|
导读:美国FDA批准Nplate(romiplostim)作为首个并且是唯一的血小板生成药物,用于治疗脾切除及非脾切除的成人免疫性血小板减少症。Nplate为首个FDA批准的肽体蛋白,通过升高与维持血小板数而发挥作用,是长期治疗该慢性疾病的新方法。
美国FDA批准Nplate(romiplostim)用于成人慢性免疫性血小板减少症ITP的长期治疗。
FDA Approves Nplate for Long-Term Treatment of Adult Chronic ITP
美国FDA批准Nplate(romiplostim)作为首个并且是唯一的血小板生成药物,用于治疗脾切除及非脾切除的成人免疫性血小板减少症。Nplate为首个FDA批准的肽体蛋白,通过升高与维持血小板数而发挥作用,是长期治疗该慢性疾病的新方法。
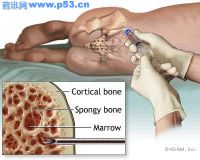
慢性ITP是1种严重的自身免疫性疾病,其特征为血液中血小板计数低(血小板减少症),可能导致严重出血。
本品的批准是基于2项双盲临床研究,纳入患者约125例,这些患者先前至少接受过1次ITP治疗。其中1项研究纳入的患者未进行脾切除,另1项中的患者则无脾。在为期6个月的研究中,接受本品治疗的患者,其血小板计数明显增高,并能维持。未进行脾切除的患者对本品的反应要高于脾切患者。
在临床研究中,与本品相关的严重不良反应有骨髓网硬蛋白沉积、停用后血小板减少症恶化。其他用药风险包括由于血小板过度升高而引起血凝块。另外,如果患者骨髓发育不良而服用本品,可能引起急性白血病。针对本品的治疗风险,已开展“风险评估和减缓策略”(REMS)。
NPLATE Company:
Amgen
Pharmacologic class:
Thrombopoietin receptor agonist
Active ingredients:
Romiplostim (recombinant) 250mcg, 500mcg; per vial; lyophilized pwd for SC inj after reconstitution; contains sucrose and mannitol; reservative-free.
Indication:
Thrombocytopenia in patients with chronic immune (idiopathic) thrombocytopenic purpura (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Pharmacology:
Nplate is a member of the thrombopoietin (TPO) mimetic class. It is an Fc-peptide fusion protein that activates intracellular transcriptional pathways leading to increased platelet production via the TPO receptor.
Nplate produces dose-dependent increases in platelet production by binding and activating the TPO receptor in a mechanism similar to endogenous TPO.
Clinical Trials:
The safety and efficacy of Nplate was evaluated in 2 open-label, placebo-controlled clinical trials of chronic ITP patients with or without splenectomies and platelet counts ≤30x109/L. Patients in both studies receivedweeklySCinjectionsindividually adjusted to maintain platelet counts between 50 to 200x109/L.
Splenectomized patients achieved an overall platelet response of 79versus 0%, when compared to placebo. Non-splenectomized patients achieved an overall platelet response of 88% versus 14%, when compared to placebo. A reduction or discontinuation of baseline concurrent ITP treatments were also seen in both groups compared to placebo.
Adults:
Give by SC inj. To reduce risk of bleeding: use lowest effective dose to achieve and maintain platelets ≥50x109/L. ≥18yrs: initially: 1mcg/kg weekly; may increase by 1mcg/kg if platelets <50x109/L; max: 10mcg/kg weekly. May reduce by 1mcg/kg if platelets >200x109/L for 2 consecutive weeks. Do not dose if platelets >400x109/L; resume Nplate at a dose reduced by 1mcg/kg when platelets fallen to <200x109/L. Discontinue if platelets has not increased after 4 weeks at max dose.
Children:
<18yrs: not recommended.
Precautions:
Not for normalization of platelet counts. Risk of bone marrow fibrosis with cytopenias. Worsened thrombocytopenia after discontinuation. Monitor CBCs, platelets, and peripheral blood smears before and weekly during dose adjustments then monthly after achieving stable dose; and weekly for 2 weeks after discontinuation of therapy. Monitor after initial response for formation of neutralizing antibodies. Risk of hematologic malignancies (esp. myelodysplastic syndrome). Renal or hepatic impairment. Elderly. Pregnancy (Cat.C). Nursing mothers.
Interactions:
May increase bleeding risk with anticoagulants or antiplatelet agents.
Adverse reactions:
Arthralgia, dizziness, insomnia, myalgia, pain in extremity, abdominal pain, shoulder pain, dyspepsia, paresthesia, headaches; bone marrow reticulin deposition, worsening thrombocytopenia, risk of bleeding, thrombotic/thromboembolic complications, antibody formation.
Notes:
Available only through Nplate NEXUS program. To register for Nplate NEXUS program or for pregnancy registry call Amgen at (877) Nplate1.
How supplied:
Single-use vials?
Last Updated:
10/10/08 |