|
普卡必利可用于治疗慢性便秘对于缓泻药治疗不满意者亦有效 比利时学者塔克(Tack)的一项研究结果显示,普卡必利可显著且持续地增强慢性便秘患者的肠功能,改善相关症状,提高患者的满意程度。这篇论文发表在《消化道》[Gut 2009,58(3):357]杂志上。 这项多中心、随机、安慰剂对照的平行Ⅲ期试验纳入716例慢性便秘[定义为自发全肠运动(SCBM)≤2次/周]患者,给予普卡必利2 mg或普卡必利4 mg 或安慰剂治疗,每天一次,持续12周。研究者评价获得SCBM≥3次/周的患者比例及SCBM增加≥1次/周的患者比例,同时评估患者满意程度、不良反应事件等。 结果显示,共713例患者接受所有项目评价。治疗超过平均12周后,19.5%的普卡必利 2 mg组患者(P<0.01)和23.6%的4 mg组患者(P<0.001)获得SCBM≥3次/周,显著高于安慰剂组(9.6%),在以前接受缓泻药治疗不满意的患者亚组中,接受普卡必利治疗者疗效亦优于安慰剂组。 此外,接受普卡必利两种剂量治疗的患者其满意程度较高,SCBM增加≥1次/周者增多。普卡必利治疗后最常见的相关不良事件是头痛和腹泻,但这两种剂量都是安全的,且患者耐受性良好。 《新英格兰医学杂志》美国罗切斯特梅奥医院报道,采用普卡必利治疗重度慢性便秘短期可显著改善肠道功能,减轻便秘症状。 普卡必利(prucalopride)具有高度选择性及特异性的5-HT4受体激动作用。 Camilleri等进行了一项多中心、随机对照研究,对620名重度慢性便秘(大便次数≤2次/周)分别每天服用安慰剂、普卡必利2mg或4mg,疗程12周。结果三组排便次数增加的比例分别为25.8%、47.3%和46.6%。普卡必利治疗的两组患者对治疗的满意度和症状严重程度认知都有显著改善,并未发现严重的心血管影响。 虽然短期服用普卡必利可显著减轻便秘症状,但研究者认为还有待长期、大规模研究,进一步评估该药的风险和受益。 Prucalopride治疗脊髓损伤引起的慢性便秘有效 丹麦外科研究所Jensen等报告prucalopride治疗脊髓损伤引起的慢性便秘有较好疗效,病人亦能耐受。 脊髓损伤病人常常有慢性便秘,prucalopride是一种新的高选择性、特异性5羟色胺受体激动剂,有促进肠蠕动的作用。Jensen等进行了一项双盲、安慰剂对照、Ⅱ期剂量递增研究,对prucalopride治疗脊髓损伤引起的慢性便秘的疗效和耐受性进行了研究。(ScandJGastroenterol 2002,37 ∶431) 在4周试运行期后,8例病人用prucalopride 1 mg,4例病人用安慰剂;11例新病人随机分为2组,分别用prucalopride 2 mg(8例)或安慰剂(3例)。每日服药1次,疗程4周。每日记录排便次数,评估便秘严重性和治疗效果[采用视觉模拟评分法(VAS),0~100 mm],测定结肠通过时间。 结果显示,和试运行期相比,安慰剂组病人便秘加重,prucalopride 1 mg组和2 mg组病人便秘减轻。VAS显示,疗效与剂量显著相关(安慰剂、1 mg和2 mg组病人VAS中位数分别为4、52和73)。在4周观察期间,2 mg组病人的平均每周排便次数有所增加(中位数为0.6,95%CI为0.2~1.2),2 mg组病人结肠通过时间中位数显著缩短。4例病人(2 mg组)有中重度腹痛,其中2例病人中断治疗。治疗对各项安全参数无显著影响。 该研究显示,prucalopride对治疗脊髓损伤引起的慢性便秘可能有重要作用。 Prucalopride可治疗胃肠道硬皮病 比利时和荷兰研究人员报道,Janssen制药公司的Prucalopride(Ⅰ)成功解决2例患者与胃肠道硬皮症有关的症状。 他们报道的2例女患者(年龄分别为30岁和60岁)患有长期硬皮病。两位患者都有胃肠道硬皮病、特异性吞咽困难、早期饱满感、腹胀、便秘等症状。通过食管和小肠测压法证实胃肠道动力异常。当患者采用常规疗法没有获得足够的疗效时,他们即开始接受每日2mg的(Ⅰ)。经(Ⅰ)治疗后,胃肠蠕动性得以改善,食欲增加,腹胀减轻,其他胃肠活动异常恢复正常。据称,该疗法在两个病人中成功持续1年以上。 Resolor® (prucalopride) Innovative Compound for Chronic ConstipationOur lead compound, prucalopride, is indicated for the treatment of chronic constipation (CC) in patients who are not adequately relieved by laxative treatments. Prucalopride is a novel, very selective small molecule that stimulates certain sites (5-HT4 receptor agonists) in the GI tract in a specific manner. As a result, it increases movement of the colon and stimulates mass movement in the bowels in a physiologic way. Also see movie file at the bottom of the page Prucalopride is currently under review by the European Medicines Evaluation Agency (EMEA) since June 2008 and Swissmedic since July 2008. When approved, prucalopride will be the first in a class of highly selective, high-affinity 5-HT4 receptor agonists with a favourable benefit/safety ratio, with the potential to improve the symptoms of people with severe chronic constipation. Potential Market
Development StatusThe marketing authorization application we filed for prucalopride includes a comprehensive clinical-development program of three extensive and identically designed pivotal Phase III studies in the target indication (i.e., the treatment of chronic constipation in adults not adequately relieved by laxatives). Total prucalopride exposure in the program exceeds 3,000 patients. Once approved the company intends to develop the product in Opiod Induced Constipation (OIC). |
|
当前位置:药品说明书与价格首页 >> 综合药讯 >> 普卡必利可用于治疗慢性便秘
普卡必利可用于治疗慢性便秘简介:
普卡必利可用于治疗慢性便秘对于缓泻药治疗不满意者亦有效
比利时学者塔克(Tack)的一项研究结果显示,普卡必利可显著且持续地增强慢性便秘患者的肠功能,改善相关症状,提高患者的满意程度。这篇论文 ... 责任编辑:admin
|
最新文章更多推荐文章更多热点文章更多
|


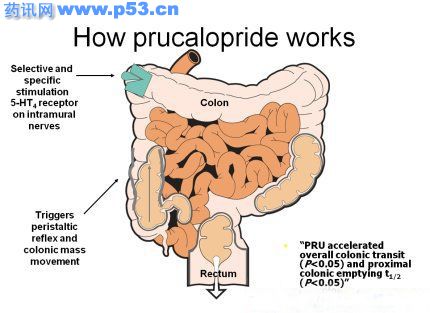
 Chronic Constipation is a disorder of the gastrointestinal tract. It is a prevalent and debilitating condition that is not always well understood and, in many cases, is inadequately treated. In Europe, an estimated 11 million patients frequently visit their doctor with complaints of constipation after unsatisfactory results with over-the-counter medication or prescription laxatives. More than 50% of patients remain dissatisfied.
Chronic Constipation is a disorder of the gastrointestinal tract. It is a prevalent and debilitating condition that is not always well understood and, in many cases, is inadequately treated. In Europe, an estimated 11 million patients frequently visit their doctor with complaints of constipation after unsatisfactory results with over-the-counter medication or prescription laxatives. More than 50% of patients remain dissatisfied. These three Phase III studies demonstrated that, over a 12-week treatment period, prucalopride significantly improved bowel performance and associated symptoms. Additional data show that, over a two-year period of testing, prucalopride has maintained a strong efficacy and safety profile with patients reporting high levels of satisfaction in terms of the overall functioning of their bowels and this in both short and long term treatment. Studies performed in elderly showed that the product is also effective in this patient population.
These three Phase III studies demonstrated that, over a 12-week treatment period, prucalopride significantly improved bowel performance and associated symptoms. Additional data show that, over a two-year period of testing, prucalopride has maintained a strong efficacy and safety profile with patients reporting high levels of satisfaction in terms of the overall functioning of their bowels and this in both short and long term treatment. Studies performed in elderly showed that the product is also effective in this patient population.