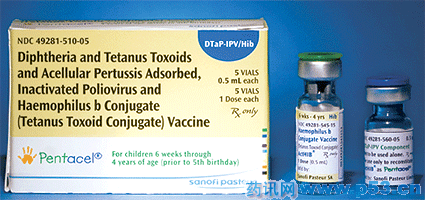
美国FDA批准首个5合1儿童新复方疫苗Pentacel上市,用于婴儿和出生6周~4岁儿童对白喉、破伤风、百日咳、脊髓灰质炎及Hib流感的免疫。本品由赛诺菲·安万特集团(sanofi-aventis Group)疫苗子公司赛诺菲巴斯德公司(Sanofi Pasteur)开发,以白喉、破伤风类毒素、吸附的无细胞百日咳、灭活的脊髓灰质炎和与破伤风类毒素结合的Hib流感病毒制成的疫苗。
本品获准采用4剂给药方案:在出生后2、4、6和15~18月各1次。按照目前美国疾病预防和控制中心推荐的儿童免疫方案,出生后18个月使用单种疫苗需注射多达23次。本品通过减少注射次数简化免疫给药方案。
美国FDA批准本品上市基于在美国和加拿大纳入5 000多例儿童、每个儿童至少注射1剂Pentacel疫苗的多中心临床研究结果。本品的免疫性和安全性可与分别接种5种相应的单一疫苗相媲美。
在临床研究中,注射本品后报告的局部和全身性不良反应很少,与分别注射单一疫苗相近。本品最常见的不良反应是注射部位红肿和触痛,发热,激动和哭叫。
DTaP-IPV/Hib vaccine for children
Company:
Sanofi Pasteur
Vaccine (DTaP-IPV + Hib/PRP-T)
Diphtheria and tetanus toxoids + acellular pertussis adsorbed + inactivated poliovirus vaccine (susp); Hib conjugate (tetanus toxoid conjugate, PRP) (lyophilized pwd); 0.5mL/dose; for IM inj after mixing; contains residual trace amounts of formaldehyde, 2-phenoxy-ethanol, neomycin, polymyxin B.
Active immunization against diphtheria, tetanus, pertussis, poliomyelitis and invasive disease due to Haemophilus influenze type b in infants and children 6 weeks through 4 years of age (prior to 5th birthday).
Pentacel is a combination vaccine that can replace the separate injections of DTaP, IPV and Hib conjugate vaccines that are recommended for children through 2 years of age. It was developed to align well with the existing childhood vaccination schedule. Pentacel contains the same five acellular pertussis antigens as Daptacel (DTaP, also from Sanofi Pasteur), but Pentacel has a higher concentration of two of the antigens.
Pentacel was approved based on studies that established its immunogenicity for the five separate components compared to those of the separately-administered vaccines that are the current standard of care in the US: Daptacel (DTaP), IPOL (trivalent inactivated polio-virus vaccine), and ActHIB (Hib/PRP-T). A bridging study was also used to support the conclusion that Pentacel will provide protection against pertussis disease comparable to that established for Daptacel. Pentacel may be given concurrently with other vaccines (eg, MMR, varicella, hepatitis B, 7-valent pneumococcal conjugate vaccines).
Not recommended.
Each dose is 0.5mL IM, in anterolateral thigh (infants) or deltoid muscle. Give as a 4 dose series at 2 months, 4 months, 6 months, and 15?8 months of age. May give 1st dose as early as 6 weeks of age. Previously vaccinated with ≥1 dose of Daptacel, IPV, or Hib conjugate vaccine: Pentacel may be used to complete vaccination series; see literature. May give other vaccines concurrently (use separate inj site).
Anaphylaxis associated with previous dose or component. Encephalopathy within 7 days of a previous dose of a pertussis-containing vaccine. Progressive neurological disorder (eg, infantile spasms, uncontrolled epilepsy, progressive encephalopathy).
Fever (≥105癋 within 48hrs), persistent inconsolable crying (lasting ≥3hrs within 48hrs), shock (within 48hrs), convulsions (within 3 days), or Guillain-Barre syndrome (within 6 weeks) of previous pertussis vaccine. Seizure risk (may pretreat with antipyretics, eg, acetaminophen). May defer in acute febrile illness. Have epinephrine (1:1000) available. Immune deficiency. Pregnancy (Cat.C): not recommended.
Immunosuppressants: may get suboptimal response.
Local reactions: injection site erythema, swelling, tenderness. Systemic reactions: fever, crying, fussiness.
Report adverse events to VAERS at (800) 822-7967 and to Sanofi Pasteur at (800) 822-2463.
Doses? (2 vials/dose)