发布日期:2008-08-12 21:23:15 作者: 来源: 互联网 浏览次数: 0 文字大小:【 大】【 中】【 小】
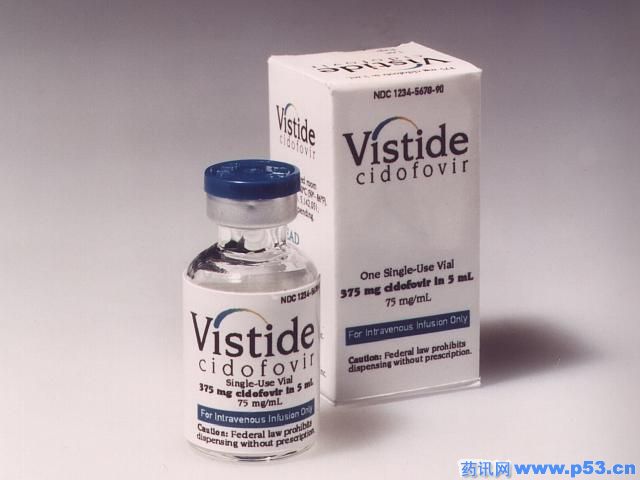
| VISTIDE |
| Manufacturer |
| Gilead Sciences, Inc. |
| Legal Classification |
| Rx |
| Pharmacological Class |
| Nucleotide analogue. |
| Generic Name |
| Cidofovir 75mg/mL; for IV infusion after dilution; preservative-free. |
| Indications |
| Treatment of cytomegalovirus (CMV) retinitis in patients with AIDS. |
| Children |
| Not recommended. |
| Adults |
| Give by IV infusion over 1hr. Pretreat with oral probenecid (2g three hrs before starting cidofovir dose, and 1g two and eight hrs after cidofovir infusion has ended) and IV normal saline hydration (1L immediately before each dose of cidofovir; patients who can tolerate fluid load should receive a 2nd liter either during or after each dose of cidofovir). Induction: 5mg/kg once weekly for 2 consecutive weeks. Maintenance: 5mg/kg once every 2 weeks; reduce to 3mg/kg if serum creatinine increases 0.3–0.4mg/dL above baseline. Discontinue if serum creatinine increases ≥0.5mg/dL above baseline or if ≥3+ proteinuria develops. |
| Contraindications |
| Serum creatinine >1.5mg/dL or CrCl ≤55mL/min, or urine protein ≥100mg/dL. Severe probenecid or sulfa allergy. Within 7 days of discontinuing other nephrotoxic drugs (e.g., amphotericin B, aminoglycosides, foscarnet, IV pentamidine, NSAIDs, vancomycin). Not for intraocular injection. |
| Precautions |
| Do not exceed recommended dose, frequency, or rate of administration (increased risk of nephrotoxicity). Women should use effective contraception during and one month after therapy. Men should use barrier contraception during and 3 months after therapy. Monitor serum creatinine, urine protein, WBC with differential before each dose; monitor intraocular pressure, visual acuity, ocular symptoms and for tinnitus. Handle and dispose of properly. Elderly. Pregnancy (Cat.C), nursing mothers: not recommended. |
| Interactions |
| See Contraindications. Probenecid interferes with the metabolism or renal tubular secretion of many drugs (including zidovudine, adjust dose). |
| Adverse Reactions |
| Elevated serum creatinine, proteinuria, neutropenia, ocular hypotony, uveitis, iritis, metabolic acidosis, GI disturbances, fever, asthenia, rash, headache, alopecia, infection, chills, dyspnea; others: see literature. |
| How Supplied |
| Single-use vials (5mL)—1 |
| Additional Resources | |
|
最新图片
|