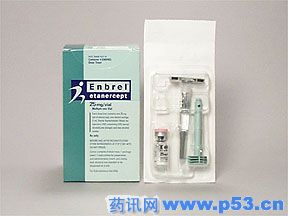
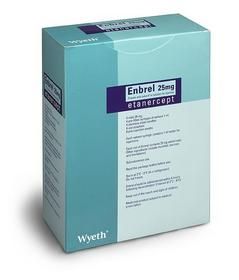
欧盟批准银屑病药50mg Enbrel上市销售
欧盟委员会已批准惠氏银屑病治疗药Enbrel(50毫克),该药每周用药一次,中-重度银屑病患者均可使用。至此,它将取代目前常用的25毫克Enbrel,这种剂量的药物每周需要用药2次。
惠氏在欧洲对50毫克Enbre进行了相关的临床实验,以观察其治疗效果。这次能通过欧盟的批准,正是以这些临床实验结果为依据。
这次实验的主要目的之一是检测Enbre能否将患者银屑病病变面积和严重程度指数至少降低75%,同行还对患者的生活质量进行评估。第一阶段实验为期12周,受试者分为两组,一组用Enbre治疗,另一组则使用安慰剂。
经过12周的治疗之后,Enbre受试组患者将进入下一个开放标签实验阶段,疗程也为12周。实验进行到12周时,主要的检测项目均达到预期目标,而到24周时,重症患者严重的皮肤损伤显著减少,64%的病变面积变得“光滑”或“近乎光滑”。
ENBREL 25MG/VIAL FOR INJECTION
Drug Information For: ENBREL 25MG/VIAL FOR INJECTION
Ingredient Name: ETANERCEPT (et-a-NER-sept)
Drug Manufacturer: AMGEN
Common Uses: This medicine is an immune response modifier used to reduce the signs and symptoms of rheumatoid or psoriatic arthritis, juvenile arthritis, ankylosing spondylitis, or moderate to severe psoriasis. Etanercept is also used to stop the progression of damage caused by psoriatic arthritis. It may be used to treat other conditions as determined by your doctor.
Before Using This Medicine: WARNING: SEVERE AND SOMETIMES FATAL INFECTIONS (such as bacterial sepsis, tuberculosis) have occurred in patients using this medicine. Tuberculosis (TB) may be caused by a new infection or by reactivation of a previous infection. Your doctor will test you for TB and evaluate your risk for developing it. This will occur before and during treatment with this medicine. If you have TB, you should begin to treat it before you begin treatment with this medicine. CONTACT YOUR DOCTOR RIGHT AWAY if you develop fever, chills, or sore throat; unusual nausea, vomiting, stomach pain, or diarrhea; fast heartbeat; decreased mental alertness; rapid breathing; new or worsening cough; shortness of breath; chest pain or discomfort; swelling of the lymph nodes; or general feeling of being unwell. Some medicines or medical conditions may interact with this medicine. INFORM YOUR DOCTOR OR PHARMACIST of all prescription and over-the-counter medicine that you are taking. ADDITIONAL MONITORING OF YOUR DOSE OR CONDITION may be needed if you are taking anakinra. DO NOT START OR STOP any medicine without doctor or pharmacist approval. Inform your doctor of any other medical conditions, including uncontrolled bleeding, recurring or ongoing infections, histoplasmosis, lupus, a central nervous system demyelinating disorder (such as multiple sclerosis, myelitis, optic neuritis), a history of lymphoma or cancer, heart disease (such as heart failure), allergies, pregnancy, or breast-feeding. TELL YOUR DOCTOR if you have a history of TB, positive TB skin test, or close contact with someone who has TB. USE OF THIS MEDICINE IS NOT RECOMMENDED if you have any infection. Use of this medicine in children under age 2 is not recommended. Discuss with your doctor the risks and benefits of giving this medicine to your child. Contact your doctor or pharmacist if you have any questions or concerns about taking this medicine.
How to Use This Medicine: Follow the directions for using this medicine provided by your doctor. This medicine is administered as an injection at your doctor's office, hospital, or clinic. This medicine is sometimes used at home as an injection. If you are using this medicine at home, a healthcare professional will provide detailed instructions for its appropriate use. Ask any questions that you may have about this medicine or giving injections. DO NOT SHAKE this medicine. DO NOT USE THIS MEDICINE if it is cloudy or discolored. Rotate the site where the medicine is injected. Do not inject into areas where the skin is hard, tender, bruised, red, or irritated in any way. STORE THIS MEDICINE in the refrigerator between 36 and 46 degrees F (2 and 8 degrees C). Do not freeze. After mixing, the medicine may be stored in the refrigerator between 36 and 46 degrees F (2 and 8 degrees C) for up to 14 days. Discard solutions mixed for more than 14 days. IF YOU MISS A DOSE OF THIS MEDICINE, contact your doctor to establish a new dosing schedule.
Cautions: DO NOT TAKE THIS MEDICINE if you have had an allergic reaction to it or are allergic to any ingredient in this product. IT MAY TAKE SEVERAL WEEKS for this medicine to work. Do not stop using this medicine without checking with your doctor. KEEP ALL DOCTOR AND LABORATORY APPOINTMENTS while you are taking this medicine. BEFORE YOU HAVE ANY MEDICAL OR DENTAL TREATMENTS, EMERGENCY CARE, OR SURGERY, tell the doctor or dentist that you are using this medicine. THIS MEDICINE MAY LOWER YOUR RESISTANCE TO INFECTION. Prevent infection by avoiding contact with people with colds or other infections. Do not touch your eyes or the inside of your nose unless you have thoroughly washed your hands first. Contact your doctor right away if you develop signs of an infection such as fever, chills, or sore throat; unusual nausea, vomiting, stomach pain, or diarrhea; fast heartbeat; decreased mental alertness; rapid breathing; new or worsening cough; shortness of breath; chest pain or discomfort; swelling of the lymph nodes; or general feeling of being unwell. THIS MEDICINE MAY REDUCE THE NUMBER OF BLOOD CELLS THAT ARE NEEDED FOR CLOTTING. To prevent bleeding, avoid situations where bruising or injury may occur. THIS MEDICINE MAY INCREASE THE RISK OF DEVELOPING LYMPHOMA (BLOOD CANCER) OR OTHER TYPES OF CANCER. Discuss any questions or concerns with your doctor. Tell your doctor if you have ever had cancer. Contact your doctor right away if you develop symptoms of lymphoma such as unusual lumps or swelling (eg, in your neck, armpit, or groin), night sweats, recurring fever, unusual tiredness or weakness, persistent unexplained itching, or unexplained weight loss. CHECK WITH YOUR DOCTOR BEFORE HAVING IMMUNIZATIONS (VACCINATIONS) while you are using this medicine. IF YOU HAVE NOT HAD CHICKENPOX OR MEASLES, avoid contact with anyone who has any of these diseases. BEFORE YOU BEGIN TAKING ANY NEW MEDICINE, either prescription or over-the-counter, check with your doctor or pharmacist. FOR WOMEN: IF YOU PLAN ON BECOMING PREGNANT, discuss with your doctor the benefits and risks of using this medicine during pregnancy. IT IS UNKNOWN IF THIS MEDICINE IS EXCRETED in breast milk. DO NOT BREAST-FEED while taking this medicine.
Possible Side Effects: SIDE EFFECTS that may occur while taking this medicine include pain, redness, itching, bruising, or swelling at the injection site; headache; or dizziness. If they continue or are bothersome, check with your doctor. CONTACT YOUR DOCTOR IMMEDIATELY if you develop a cold or any other infection; fever, chills, or sore throat; unusual nausea, vomiting, stomach pain, or diarrhea; fast heartbeat; decreased mental alertness; rapid breathing; new or worsening cough; shortness of breath; chest pain or discomfort; swelling of the lymph nodes; general feeling of being unwell; butterfly rash (rash on nose and cheeks); unusual tiredness or weakness; unusual bruising or bleeding; numbness or tingling sensations; vision changes; pale skin; or unusual lumps. AN ALLERGIC REACTION to this medicine is unlikely, but seek immediate medical attention if it occurs. Symptoms of an allergic reaction include rash, itching, swelling, dizziness, or trouble breathing. If you notice other effects not listed above, contact your doctor, nurse, or pharmacist.
Overdose: If overdose is suspected, contact your local poison control center or emergency room immediately.
Additional Information: YOU MAY SEE IMPROVEMENT OF YOUR SYMPTOMS as soon as 1 to 2 weeks after starting this medicine. Up to 3 months of treatment may be needed for some patients. DO NOT SHARE THIS MEDICINE with others for whom it was not prescribed. DO NOT USE THIS MEDICINE for other health conditions. KEEP THIS PRODUCT, as well as syringes and needles, out of the reach of children. Do not reuse needles, syringes, or other materials. Dispose of properly after use. Ask your doctor, nurse, or pharmacist to explain local regulations for selecting an appropriate container and properly disposing of the container when it is full.