|
英文药名:REGRANEX(becaplermin Gel 0.01%) 中文药名:贝卡普勒明凝胶 生产厂家:杨森制药公司
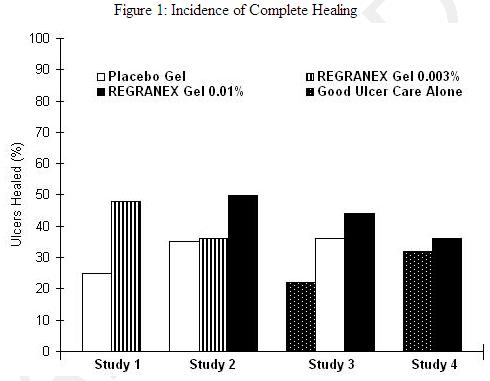
In a randomized, double-blind study of REGRANEX Gel (100 mcg/g once daily for 16 weeks) in patients with Stage III or IV pressure ulcers, the incidence of complete ulcer closure was 15% (28/189) in the becaplermin group and 12% (22/190) in the vehicle control group. This difference was not statistically significant. In two small, randomized, double-blinded studies of REGRANEX Gel (100mcg/g once daily for 16 weeks) in patients with venous stasis ulcers, the combined incidence of complete ulcer closure was 46% (30/65) in the becaplermin group and 39% (26/67) in the vehicle control group. This difference was not statistically significant. INDICATIONS AND USAGE REGRANEX (becaplermin) Gel is indicated for the treatment of lower extremity diabetic neuropathic ulcers that extend into the subcutaneous tissue or beyond and have an adequate blood supply. When used as an adjunct to, and not a substitute for, good ulcer care practices including initial sharp debridement, pressure relief and infection control, REGRANEX Gel increases the incidence of complete healing of diabetic ulcers. The efficacy of REGRANEX Gel has not been established for the treatment of pressure ulcers and venous stasis ulcers (see Clinical Studies) and has not been evaluated for the treatment of diabetic neuropathic ulcers that do not extend through the dermis into subcutaneous tissue (Stage I or II, IAET staging classification) or ischemic diabetic ulcers. CONTRAINDICATIONS REGRANEX (becaplermin) Gel is contraindicated in patients with: -known hypersensitivity to any component of this product (e.g., parabens); -known neoplasm(s) at the site(s) of application. WARNINGS REGRANEX Gel contains becaplermin, a recombinant human platelet-derived growth factor, which promotes cellular proliferation and angiogenesis. (See Clinical Pharmacology). The benefits and risks of becaplermin treatment should be carefully evaluated before prescribing. Becaplermin should be used with caution in patients with a known malignancy. Malignancies distant from the site of application have occurred in becaplermin users in both a clinical study and in postmarketing use, and an increased rate of death from systemic malignancies was seen in patients who have received 3 or more tubes of REGRANEX Gel. In a follow-up study, 491 (75%) of 651 subjects from two randomized, controlled trials of becaplermin gel 0.01% were followed for a median of approximately 20 months to identify malignancies diagnosed after the end of the trials. Eight of 291 subjects (3%) from the becaplermin group and two of 200 subjects (1%) from the vehicle/standard of care group were diagnosed with cancers during the follow-up period, a relative risk of 2.7, (95% confidence interval 0.6–12.8). The types of cancers varied and all were remote from the treatment site. In a retrospective study of a medical claims database, cancer rates and overall cancer mortality were compared between 1,622 patients who used REGRANEX Gel and 2,809 matched comparators. Estimates of the incidence rate reported below may be under-reported due to limited follow-up for each individual. The incidence rate for all cancers was 10.2 per 1,000 person years for patients treated with REGRANEX Gel and 9.1 per 1,000 person years for the comparators. Adjusted for several possible confounders, the rate ratio was 1.2, (95% confidence interval 0.7–1.9). Types of cancers varied and were remote from the site of treatment. The incidence rate for mortality from all cancers was 1.6 per 1,000 person years for those who received REGRANEX Gel and 0.9 per 1,000 person years for the comparators. The adjusted rate ratio was 1.8 (95% confidence interval 0.7–4.9). The incidence rate for mortality from all cancers among patients who received 3 or more tubes of REGRANEX Gel was 3.9 per 1,000 person years and 0.9 per 1,000 person years in the comparators. The adjusted rate ratio for cancer mortality among those who received 3 or more tubes relative to those who received none was 5.2, (95% confidence interval 1.6–17.6). (See Boxed Warning) REGRANEX Gel is a non-sterile, low bioburden preserved product. Therefore, it should not be used in wounds that close by primary intention. PRECAUTIONS For external use only. If application site reactions occur, the possibility of sensitization or irritation caused by parabens or m-cresol should be considered. The effects of becaplermin on exposed joints, tendons, ligaments, and bone have not been established in humans. In preclinical studies, rats injected at the metatarsals with 3 or 10 µg/site (approximately 60 or 200 µg/kg) of becaplermin every other day for 13 days displayed histological changes indicative of accelerated bone remodeling consisting of periosteal hyperplasia and subperiosteal bone resorption and exostosis. The soft tissue adjacent to the injection site had fibroplasia with accompanying mononuclear cell infiltration reflective of the ability of PDGF to stimulate connective tissue growth. Information for Patients Patients should be advised that: –hands should be washed thoroughly before applying REGRANEX Gel; –the tip of the tube should not come into contact with the ulcer or any other surface; the tube should be recapped tightly after each use; –a cotton swab, tongue depressor, or other application aid should be used to apply REGRANEX Gel; –REGRANEX Gel should only be applied once a day in a carefully measured quantity (see Dosage and Administration section). The measured quantity of gel should be spread evenly over the ulcerated area to yield a thin continuous layer of approximately 1/16 of an inch thickness. The measured length of the gel to be squeezed from the tube should be adjusted according to the size of the ulcer. The amount of REGRANEX Gel to be applied daily should be recalculated at weekly or biweekly intervals by the physician or wound care giver; Step-by-step instructions for application of REGRANEX Gel are as follows: Squeeze the calculated length of gel on to a clean, firm, non-absorbable surface, e.g., wax paper. With a clean cotton swab, tongue depressor, or similar application aid, spread the measured REGRANEX Gel over the ulcer surface to obtain an even layer. Cover with a saline moistened gauze dressing. –after approximately 12 hours, the ulcer should be gently rinsed with saline or water to remove residual gel and covered with a saline-moistened gauze dressing (without REGRANEX Gel); –it is important to use REGRANEX Gel together with a good ulcer care program, including a strict non-weight-bearing program; –excess application of REGRANEX Gel has not been shown to be beneficial; –REGRANEX Gel should be stored in the refrigerator. Do not freeze REGRANEX Gel; –REGRANEX Gel should not be used after the expiration date on the bottom, crimped end of the tube. Drug Interactions It is not known if REGRANEX Gel interacts with other topical medications applied to the ulcer site. The use of REGRANEX Gel with other topical drugs has not been studied. Carcinogenesis, Mutagenesis, Impairment of Fertility Becaplermin was not genotoxic in a battery of in vitro assays, (including those for bacterial and mammalian cell point mutation, chromosomal aberration, and DNA damage/repair). Becaplermin was also not mutagenic in an in vivo assay for the induction of micronuclei in mouse bone marrow cells. Carcinogenesis and reproductive toxicity studies have not been conducted with REGRANEX Gel. Pregnancy Category C Animal reproduction studies have not been conducted with REGRANEX Gel. It is also not known whether REGRANEX Gel can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. REGRANEX Gel should be given to pregnant women only if clearly needed. Nursing Mothers It is not known whether becaplermin is excreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when REGRANEX Gel is administered to nursing women. Geriatric Use Among patients receiving any dose of REGRANEX Gel in clinical studies of diabetic lower extremity ulcers, 150 patients were 65 years of age and older. No overall differences in safety or effectiveness were observed between patients < 65 years of age and patients ≥ 65 years of age. The number of patients aged 75 and older were insufficient (n=34) to determine whether they respond differently from younger patients. Pediatric Use Safety and effectiveness of REGRANEX Gel in pediatric patients below the age of 16 years have not been established. ADVERSE REACTIONS Patients receiving REGRANEX (becaplermin) Gel, placebo gel, and good ulcer care alone had a similar incidence of ulcer-related adverse events such as infection, cellulitis, or osteomyelitis. However, erythematous rashes occurred in 2% of patients treated with REGRANEX Gel and placebo, and none in patients receiving good ulcer care alone. The incidence of cardiovascular, respiratory, musculoskeletal and central and peripheral nervous system disorders was not different across all treatment groups. Patients treated with REGRANEX Gel did not develop neutralizing antibodies against becaplermin. DOSAGE AND ADMINISTRATION The amount of REGRANEX Gel to be applied will vary depending upon the size of the ulcer area. To calculate the length of gel to apply to the ulcer, measure the greatest length of the ulcer by the greatest width of the ulcer in either inches or centimeters. To calculate the length of gel in inches, use the formula shown below in Table 2, and to calculate the length of gel in centimeters, use the formula shown below in Table 3. Table 2: Formula to Calculate Length of Gel in Inches to be Applied Daily INCHES
Table 3: Formula to Calculate Length of Gel in Centimeters to be Applied Daily
The amount of REGRANEX Gel to be applied should be recalculated by the physician or wound caregiver at weekly or biweekly intervals depending on the rate of change in ulcer area. The weight of REGRANEX Gel from 15g tubes is 0.65g per inch length and 0.25g per centimeter length. To apply REGRANEX Gel, the calculated length of gel should be squeezed on to a clean measuring surface, e.g., wax paper. The measured REGRANEX Gel is transferred from the clean measuring surface using an application aid and then spread over the entire ulcer area to yield a thin continuous layer of approximately 1/16 of an inch thickness. The site(s) of application should then be covered by a saline moistened dressing and left in place for approximately 12 hours. The dressing should then be removed and the ulcer rinsed with saline or water to remove residual gel and covered again with a second moist dressing (without REGRANEX Gel) for the remainder of the day. REGRANEX Gel should be applied once daily to the ulcer until complete healing has occurred. If the ulcer does not decrease in size by approximately 30% after 10 weeks of treatment or complete healing has not occurred in 20 weeks, continued treatment with REGRANEX Gel should be reassessed. The step-by-step instructions for applying REGRANEX Gel for home administration are described under "Information for Patients". HOW SUPPLIED REGRANEX (becaplermin) Gel, supplied as a clear, colorless to straw-colored preserved gel containing 100µg of becaplermin per gram of gel, is available in multi-use tubes in the following sizes: 2g tubes NDC 0045-0810-02 15g tubes NDC 0045-0810-15 REGRANEX Gel is for external use only. Storage Store refrigerated, 2–8°C (36–46°F). DO NOT FREEZE. DO NOT USE THE GEL AFTER THE EXPIRATION DATE AT THE BOTTOM OF THE TUBE. 1):https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fd2c7d21-7b07-4ab3-8983-816ab3223771 2):https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=13535 |