|
【通用名称】拉呋替丁
【药理毒理】药理研究表明,本品为高效、长效H2受体拮抗剂,对胃酸分泌具有明显的抑制作用,能抑制组胺、五肽胃泌素、食物等引起的胃酸分泌。本品具有胃粘膜保护作用,能剂量依赖性抑制多种实验动物溃疡模型溃疡的形成,促进溃疡愈合,缓解症状,预防溃疡复发。
【药代动力学】健康男性志愿者空腹单次口服拉呋替丁10mg时,Tmax为0.8±0.1小时,Cmax为174±20ng/ml,T1/2β为3.30±0.39小时,AUC0-24hr为793±85ng·hr/ml。进食状态下Tmax明显延长,但进食对Cmax、AUC和生物利用度没有影响。空腹时口服拉呋替丁10mg,给药24小时内原形药物、代谢物M-4、M-7及M-9的尿中排泄率分别为10.9±1.5%、1.7±0.2%、7.5±0.8%及0.3±0.1%,人尿中总排泄率为给药量的20%。体外研究中,拉呋替丁主要通过细胞色素P450同功酶代谢,代谢物M-4及M-9的生成与CYP3A4的参与有关,代谢物M-7的生成与CYP3A4和CYP2D6的参与有关。在浓度为3μg/ml时,人血浆蛋白结合率为88.0±1.2%。特殊人群:高龄者:对于高龄者,肾功能正常者(Cr平均值88.0±9.4ml/min)与肾功能减低倾向者(Cr20-60ml/min,均值45.2±7.8ml/min)比较,血中浓度变化无差异。透析患者:透析患者与健康成人相比,其非透析时血中原形药物Cmax升高2倍,T1/2约延长2倍,AUC增加3倍。经血液透析,拉呋替丁被清除7-18%。儿童:尚未进行药代动力学的研究。
【适应症】本品用于慢性胃炎、胃和十二指肠溃疡的治疗。
【用法和用量】每天口服1次,每次1片。
【不良反应】副作用轻微,少数患者出现头痛、困倦、腹泻、恶心、皮肤潮红、皮疹、白细胞减少、偶见血小板减少、GOT、GPT上升等,副作用发生率低,毒性较小,耐受性良好。
【特殊人群】高龄者:对于高龄者,肾功能正常者(Cr平均值88.0±9.4ml/min)与肾功能减低倾向者(Cr20-60ml/min,均值45.2±7.8ml/min)比较,血中浓度变化无差异。
透析患者:透析患者与健康成人相比,其非透析时血中原形药物Cmax升高2倍,T1/2约延长2倍,AUC增加3倍。经血液透析,拉呋替丁被清除7-18%。
儿童:尚未进行药代动力学的研究。
【禁忌】1.禁用于对本品有过敏症的患者。2.禁用于孕妇或可能怀孕的妇女。
【注意事项】有严重肾功能障碍的患者慎用。
【规格】10mg、5mg
【贮藏】遮光,密封保存。

【原产地英文商品名】Protecadin 5mg 100tabs
【原产地英文药品名】Lafutidine
【中文参考商品译名】
提示:以下产品是不同的规格和不同的价格,购买时请以电话咨询为准
·Protecadin 5毫克/片 100片/盒
·Protecadin 10毫克/片 1000片/盒
·Protecadin 10毫克/片 100片/盒
【中文参考药品译名】拉呋替丁
【生产厂家中文参考译名】Taiho
【生产厂家英文名】Taiho
Information on Protecadin
INDICATIONS
Gastric ulcers, duodenal ulcers and stomal ulcers;Gastric mucosal lesions (erosion, hemorrhage, redness or edema) associated with acute gastritis and acute exacerbation of chronic gastritis.
DOSAGE AND ADMINISTRATION
Gastric ulcers, duodenal ulcers and stomal ulcers
For adults, the usual dosage is 10mg as lafutidine orally administered twice a day, once after breakfast and once after the evening meal or before sleeping. The dose may be adjusted according to the patient's age and symptoms.
Gastric mucosal lesions (erosion, hemorrhage, redness or edema) associated with acute gastritis and acute exacerbation of chronic gastritis
For adults, the usual dosage is 10mg as lafutidine orally administered once a day, once after the evening meal or before sleeping. The dose may be adjusted according to the patient's age and symptoms.
Preanesthetic medication
For adults, the usual dosage is 10mg as lafutidine orally administered twice, once before sleeping on the day before operation and once 2 hours before introduction of anesthetic on the day of operation.
PRECAUTIONS
Careful Administration (STOGAR should be administered with care in the following patients.)
1) Patients with a history of drug hypersensitivity
2) Patients with impaired hepatic funcition [Symptoms may be exacerbated.]
3) Patients with impaired renal funcition [Symptoms may be exacerbated.]
4) Patients on dialysis [Increase in blood concentration of lafutidine is reported]
5) The elderly
Patients should be carefully observed during treatment, and the minimum required dose should be used according to symptoms. If response is not evident, other treatments should be implemented. Careful observation should be made for any changes in hematological, hepatic or renal parameters, and for changes in other factors.
Adverse Reactions
Adverse reactions (including abnormal changes in labotaroty tests) were observed in 32 (2.5%) of the 1,287 patients evaluated at the time of approval. The main adverse reactions were constipation in 3 patients (0.2%). Abnornal changes in laboratory tests were observed in 22 patients.
1. Clinically Significant Adverse Reactions
(1) Shock, anaphylactic reactions: Shock and anaphylactic reactions may appear, therefore patients should be carefully observed. If any abnormality such as pallor facial, blood pressure decreased, redness generalized, or breathing difficult is seen, this drug should be discontinued and appropriate
measures should be taken.
(2) Hepatic function disorder (unknown frequency#),jaundice (unknown frequency#)
Hepatic function disorder involving increased AST(GOT), ALT(GPT) or gamma-GTP, or jaundice may appear.
Therefore patients should be carefully observed. If any abnormality is seen, this drug should be discontinued, and appropriate measures should be taken.
(3) Agranulocytosis (unknown frequency#), thrombocytopenia (unknown frequency#) Agranulocytosis (initial symptom: sore throat, general malaise, fever, etc.) or thrombocytopenia may occur. If any abnormality is observed, this drug should be discontinued and appropriate measures should be taken.
2. Clinically Significant Adverse Reactions (Analog)
It has been reported that pancytopenia, aplastic anemia, interstitial nephritis, oculo-muco-cutaneous syndrome (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell’s syndrome), rhabdomyolysis, heart block (atrioventricular block etc.), and asystolia may occur with other H2-receptor antagonists.
3. Other Adverse Reactions
The following adverse reactions may occur. If abnormal signs are observed, appropriate measures should be taken including reduction in the dose and discontinuation of the drug
ストガー錠5/ストガー錠10
商標名
STOGAR tab
組成
1錠中にラフチジンを10mg含有する。
*本剤は添加物として乳糖水和物、結晶セルロース、トウモロコシデンプン、軽質無水ケイ酸、クロスカルメロースナトリウム、ヒドロキシプロピルセルロース、タルク、ステアリン酸マグネシウム、ヒプロメロース、マクロゴール6000、酸化チタン、カルナウバロウを含有する。
性状
白色のフィルムコート錠でにおいはないか又はわずかに特異なにおいがある。
一般名
ラフチジン(Lafutidine)
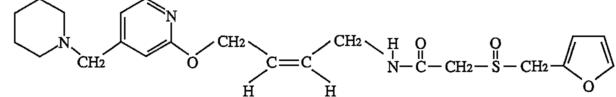
化学名
(±)-2-(Furfurylsulfinyl)-N-[4-[4-(piperidinomethyl)-2-pyridyl]oxy-(Z)-2-butenyl]acetamide
構造式
分子式
C22H29N3O4S
分子量
431.55
性状
ラフチジンは微黄白色の結晶性の粉末で、わずかに特異なにおいがある。酢酸(100)に溶けやすく、メタノールにやや溶けやすく、エタノール(99.5)にやや溶けにくく、ジエチルエーテルに極めて溶けにくく、水にほとんど溶けない。ラフチジンのメタノール溶液(1→100)は旋光性を示さない。
融点
96~99℃
分配係数
logP:-3.36(pH2)
logP:0.39(pH6)
logP:2.37(pH10)
(1-オクタノール/Britton-Robinson Buffer(20±1℃))
取扱い上の注意
〈注意〉
30℃相対湿度75%、白色蛍光灯(500Lux)8時間照射及び16時間遮光の繰り返し保存条件下において、わずかに着色することが認められたため、開封後の保存に注意すること。
包装
ストガー錠5 PTP包装:100錠(10錠×10)
ストガー錠5 PTP包装:1,000錠(10錠×100)
ストガー錠10 PTP包装:100錠(10錠×10)
ストガー錠10 PTP包装:1,000錠(10錠×100)
ストガー錠10 PTP包装:1,400錠(14錠×100)
ストガー錠10 バラ包装:500錠
|