全球第一个胰岛素笔型给药系统的制造商诺和诺德(Novo Nordisk)公司,于2004年10月8日宣布, 其Norditropin NordiFlex® (重组人生长激素注射剂,somatropin [rDNA] injection)——首个预混合、预填充式、多剂量、一次性人生长激素(hGH)笔型注射器获得FDA的批准,用于因内源性生长激素分泌不足而造成的儿童发育障碍的长期治疗。
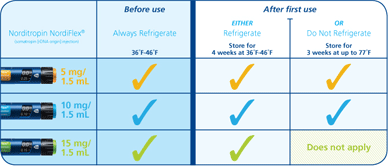
本品是在诺和诺德先进的胰岛素给药系统基础上研制而成的hGH完全整合型给药装置。本品有2种规格,给药剂量可以得到精细的调节。一种是5 mg/1.5 mL 的hGH笔型注射器,剂量范围从0.025~1.50 mg。另一种是15 mg/1.5 mL 的hGH笔型注射器,剂量范围为0.075~4.5 mg。在这两种规格的注射用笔中,有100多种不同的给药剂量可供患者选择。
本品的主要特征包括以下几点:
l使用方便:无须混合
l便于卫生保健人员培训, 护理人员和患者易于掌握使用方法
l刻度盘易于设定
l一次性,由可完全降解的塑料制成
关于Norditropin
预混合生长激素Norditropin 药筒含有通过生物合成的尽可能接近于儿童体内天然分泌的生长激素,其成份和天然生长激素完全相同。
Norditropin用于因内源性生长激素分泌不足而造成的发育障碍儿童的长期治疗,其不适用于对苯酚或者其他任何组分过敏的儿童。可能的不良反应包括头痛、肌肉痛、无力、轻微的高血糖症、尿糖、外周性水肿、体液潴留以及注射部位反应。
NORDITROPIN NORDIFLEX PEN (Somatropin) 30mg/3mL injection by Novo Nordisk
Norditropin NordiFlex Prefilled Pen available
The Norditropin NordiFlex (somatropin [rDNA origin] injection) 30mg/3mL prefilled pen has been made available by Novo Nordisk. The Norditropin NordiFlex 30mg/3mL allows children who need a growth hormone dose larger than 4.5mg to take one injection instead of two. The Norditropin NordiFlex 30mg/3mL allows the dose to be adjusted up or down as needed in increments of 0.1–6mg and can be used with NovoFine 30-gauge, NovoFine Autocover 30-gauge, and NovoFine 32-gauge Tip needles.
Norditropin NordiFlex® (somatropin [rDNA origin] injection)
*Needles may require a prescription in some states. |
| |||||||||||||||||||||||||||||||||||||||||
![]()
Norditropin® Indications and Usage
Norditropin® (somatropin [rDNA origin] injection) is used to treat children with growth failure caused by very low or no production of growth hormone. It is also used to treat children who have short stature associated with Noonan syndrome and Turner syndrome, for treatment of children with short stature born small for gestational age with no catch-up growth by age 2-4 years, and to treat adults who do not make enough growth hormone.
Important Safety Information
Remember, your doctor is the main source of information about you and your health. Please consult your doctor if you have any questions about your health or your medication.
Do not use Norditropin® if you have any of the following conditions: an allergy to phenol or any other ingredients in the medicine; active cancer or other forms of tumor; severe diabetic eye disease; acute critical illness due to certain types of heart or abdomen surgery, trauma, or acute respiratory failure.
Children should not use somatropin if they have any of the following conditions: closed epiphyses (closed bone growth plates), Prader-Willi syndrome with severe obesity, upper airway obstruction or sleep apnea, or Prader-Willi syndrome with significant respiratory impairment.
Be sure to tell your doctor if you have diabetes mellitus; have had cancer or other forms of tumor; are pregnant, planning to be pregnant or are breastfeeding.
Be sure to tell your doctor about all medications you are taking especially if they are: a glucocorticoid medication such as hydrocortisone or cortisone acetate, thyroid hormone, insulin and/or oral diabetes medicines, drugs metabolized by the liver (for example, corticosteroids, sex steroids, anticonvulsants, cyclosporine), or oral estrogen replacement.
Adult height can be influenced if you are on Norditropin® for growth failure and at the same time using glucocorticoids or thyroid hormone.
If you are treated with insulin and/or oral diabetes medicines, the dose of your insulin/oral diabetes medicines may need to be adjusted.
Side effects are usually mild and temporary. Side effects may include headaches, muscle pain, joint stiffness, weakness, high blood sugar (hyperglycemia), sugar in your urine (glucosuria), swollen hands and feet due to fluid retention, and redness and itching in the area you inject.
If you have any of these symptoms, discuss them with your doctor.
If you have headaches, eyesight problems, nausea and/or vomiting, these may be symptoms of raised pressure in the brain. Contact your doctor right away.
In very rare cases children treated with somatropin have experienced pain in the hip or knee or a limp. These symptoms may be caused by a slippage of the growth plate in the hip (slipped capital femoral epiphysis).
Scoliosis (curvature of the spine) can occur in children who experience rapid growth. Because growth hormone increases growth rate, children should be monitored for progression of scoliosis.
Thyroid function tests should be performed periodically.
Skin lesions should be checked carefully for any unusual changes.
Somatropin treatment can increase the chances of developing middle ear infections in patients with Turner syndrome.
Congenital heart disease is a common finding in Noonan syndrome and patients should be closely monitored.
Talk to your doctor if you think you have any of these conditions.
Norditropin NordiFlex® (somatropin [rDNA origin] injection)/ NordiFlex PenMate®
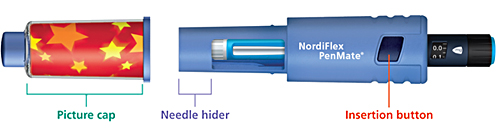
| NordiFlex PenMate®NordiFlex PenMate® helps make the injection process easy and convenient for patients. • |
The insertion button allows the needle to be automatically inserted quickly with a single touch | • |
The needle is hidden from sight during the injection process | • |
The picture cap enables the user to add pictures or drawings of his or her choice | NordiFlex PenMate® is designed for use with Norditropin NordiFlex® pens. The durable accessory slides easily over Norditropin NordiFlex® and twists and locks into place. Injection sites should always be rotated to avoid lipoatrophy. |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Norditropin® Indications and Usage
Norditropin® (somatropin [rDNA origin] injection) is indicated for the treatment of children with growth failure due to inadequate secretion of endogenous growth hormone, the treatment of children with short stature associated with Noonan syndrome and Turner syndrome, the treatment of children with short stature born small for gestational age (SGA) with no catch-up growth by age 2-4 years, and for the replacement of endogenous growth hormone in adults with growth hormone deficiency (GHD) who meet either of the following two criteria: 1. Adult Onset: Patients who have GHD, either alone or associated with multiple hormone deficiencies (hypopituitarism), as a result of pituitary disease, hypothalamic disease, surgery, radiation therapy, or trauma; or 2. Childhood Onset: Patients who were growth hormone deficient during childhood as a result of congenital, genetic, acquired, or idiopathic causes.
Important Safety Information
Somatropin should not be used for growth promotion in pediatric patients with closed epiphyses or in patients with active proliferative or severe non-proliferative diabetic retinopathy. Norditropin should not be used in patients with known hypersensitivity to somatropin or any of its excipients.
Somatropin should not be used or should be discontinued with any evidence of active malignancy. Patients with preexisting malignancy should be monitored carefully for any progression or reoccurrence.
Somatropin should not be used to treat patients with acute critical illness due to complications following open heart or abdominal surgery, multiple accidental trauma or acute respiratory failure as increased mortality may occur.
Somatropin is contraindicated in patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment. There have been reports of sudden death when somatropin was used in such patients. Norditropin® is not indicated for the treatment of patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
Blood glucose levels should be monitored periodically as treatment with somatropin may decrease insulin sensitivity. Patients with preexisting diabetes or glucose intolerance should be monitored closely during somatropin therapy. Doses of insulin or oral agents may need to be adjusted for patients with diabetes on somatropin therapy.
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea and/or vomiting has been reported in a small number of patients treated with somatropin products. Symptoms usually occurred within the first eight (8) weeks after initiation of somatropin therapy and generally resolve after cessation of therapy or a reduction of the somatropin dose. Funduscopic examination should be performed routinely before initiating and periodically during the course of somatropin therapy. If papilledema is observed by funduscopy during somatropin treatment, treatment should be discontinued.
Pediatric patients may develop slipped capital femoral epiphyses more frequently if they have endocrine disorders or during rapid growth. Any child having onset of a limp or complaints of hip or knee pain during somatropin therapy should be carefully evaluated. Progression of scoliosis can occur in patients who experience rapid growth. Somatropin has not been shown to increase the occurrence of scoliosis.
In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Patients treated with somatropin should therefore have periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted as needed.
Patients with Turner Syndrome should be evaluated carefully for otitis media and other ear disorders since these patients have an increased risk of ear and hearing disorders. Somatropin treatment may increase the occurrence of otitis media in patients with Turner syndrome. In addition, patients with Turner syndrome should be monitored closely for cardiovascular disorders (e.g., stroke, aortic aneurysm/dissection, hypertension) as these patients are also at risk for these conditions.
Although from a clinical study in Noonan syndrome there was no evidence of somatropin-induced ventricular hypertrophy or exacerbation of preexisting ventricular hypertrophy (as judged by echocardiography), the safety of Norditropin® in children with Noonan syndrome and significant cardiac disease is not known.
Somatropin inhibits 11ß-hydroxysteroid dehydrogenase type 1 (11ßHSD-1) in adipose/hepatic tissue, and may significantly impact the metabolism of cortisol and cortisone. In patients treated with somatropin, previously undiagnosed central (secondary) hypoadrenalism may be unmasked requiring glucocorticoid replacement therapy. In addition, patients treated with glucocorticoid replacement therapy especially with cortisone acetate and prednisone for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses.
Careful monitoring is advisable when somatropin is administered in combination with other drugs known to be metabolized by CP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporine) or other hormone replacement therapy.
The safety and effectiveness of Norditropin® in patients age 65 years and older has not been evaluated in clinical studies. Elderly patients may be more sensitive to the actions of somatropin and may be more prone to develop adverse reactions.
Common somatropin-related adverse reactions include injection site reactions/rashes, lipoatrophy and headaches, glucose intolerance, fluid retention and unmasking of latent central hypothyroidism.
Most serious adverse reactions reported for somatropin include intracranial hypertension, diabetic retinopathy, glucose intolerance, slipped capital femoral epiphysis, progression of preexisting scoliosis, sudden death in pediatric patients with Prader-Willi syndrome with risk factors including severe obesity, history of upper airway obstruction or sleep apnea and unidentified respiratory infection, and intracranial tumors as a 2nd tumor in patients who had been treated for a 1st neoplasm.
Please see Prescribing Information. | Download Adobe® Reader®