|
英文药名: Prepidil(Dinoprostone Gel) 中文药名: 普比迪(地诺前列酮)凝胶 生产品牌药厂家: AstraZeneca 药品介绍 局部使用前列腺素E2凝胶(地诺前列酮)广泛用于促宫颈成熟。使用前列腺素E2凝胶后,宫颈结缔组织的变化与足月分娩早期所见到的一样,包括胶原束的溶解和粘膜下层水含量的增加。使用小剂量的前列腺素E2增加了引产成功率,降低了产程延长的发生率,并减少了催产素的用量。 在1992年,FDA批准前列腺素E2凝胶(Prepidil,普比迪)用于足月或近足月有引产适应证的孕妇来促宫颈成熟。这种凝胶装在含有0.5mg地诺前列酮的2.5ml的注射器中。这种宫颈内给药的方式的优点在于:对宫缩的影响小,而对宫颈不成熟的孕妇效果较好。10mg的地诺前列酮阴道栓剂(Cervidil)在1995年也被批准用于促宫颈成熟。栓剂与凝胶相比,其药物释放更慢(0.3mg/h)。 建议这些药物在分娩时或近分娩时使用,因为此时可以进行持续性的宫缩和胎心监测。观察周期为30分钟至2小时。如果观察期间内宫缩和胎心没有变化,则病人可以被转出或出院。当宫缩发生时,通常在第一个小时宫缩比较明显,并在最初4小时内达到高峰。如果规律宫缩持续存在时,应连续监测胎心率并记录生命体征。 副作用 中文通用名称:地诺前列酮 适应症: 注意事项: 不良反应: 给药说明: 用法用量: PREPIDIL - dinoprostone gel PREPIDIL Gel contains dinoprostone as the naturally occurring form of prostaglandin E2 (PGE2) and is designated chemically as (5Z, 11a, 13E, 15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid. The molecular formula is C20H32O5 and the molecular weight is 352.5. Dinoprostone occurs as a white to off-white crystalline powder with a melting point within the range of 65° to 69°C. It is soluble in ethanol, in 25% ethanol in water, and in water to the extent of 130 mg/100 mL. The active constituent of PREPIDIL Gel is dinoprostone 0.5 mg/3 g (2.5 mL gel); other constituents are colloidal silicon dioxide NF (240 mg/3 g) and triacetin USP (2760 mg/3 g). The structural formula is represented below: 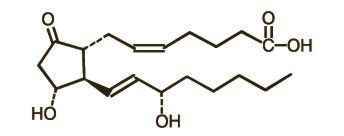 CLINICAL PHARMACOLOGY PREPIDIL Gel (dinoprostone) administered endocervically may stimulate the myometrium of the gravid uterus to contract in a manner similar to contractions seen in the term uterus during labor. Whether or not this action results from a direct effect of dinoprostone on the myometrium has not been determined. Dinoprostone is also capable of stimulating smooth muscle of the gastrointestinal tract in humans. This activity may be responsible for the vomiting and/or diarrhea that is occasionally seen when dinoprostone is used for preinduction cervical ripening. In laboratory animals, and also in humans, large doses of dinoprostone can lower blood pressure, probably as a result of its effect on smooth muscle of the vascular system. With the doses of dinoprostone used for cervical ripening this effect has not been seen. In laboratory animals, and also in humans, dinoprostone can elevate body temperature; however, with the dosing used for cervical ripening this effect has not been seen. In addition to an oxytocic effect, there is evidence suggesting that this agent has a local cervical effect in initiating softening, effacement, and dilation. These changes, referred to as cervical ripening, occur spontaneously as the normal pregnancy progresses toward term and allow evacuation of uterine contents by decreasing cervical resistance at the same time that myometrial activity increases. While not completely understood, biochemical changes within the cervix during natural cervical ripening are similar to those following PGE2-induced ripening. Further, it has been shown that these changes can take place independent of myometrial activity; however, it is quite likely that PGE2 administered endocervically produces effacement and softening by combined contraction-inducing and cervical-ripening properties. There is evidence to suggest that the changes that take place within the cervix are due to collagen degradation resulting from collagenase secretion as a response, at least in part, to PGE2. Using an unvalidated assay, the following information was determined. When PREPIDIL Gel was administered endocervically to women undergoing preinduction ripening, results from measurement of plasma levels of the metabolite 13,14-dihydro-15-keto-PGE2 (DHK-PGE2) showed that PGE2 was relatively rapidly absorbed and the Tmax was 0.5 to 0.75 hours. Plasma mean Cmax for gel-treated subjects was 433 ± 51 pg/mL versus 137 ± 24 pg/mL for untreated controls. In those subjects in which a clinical response was observed, mean Cmax was 484 ± 57 pg/mL versus 213 ± 69 pg/mL in nonresponders and 219 ± 92 pg/mL in control subjects who had positive clinical progression toward normal labor. These elevated levels in gel-treated subjects appear to be largely a result of absorption of PGE2 from the gel rather than from endogenous sources. PGE2 is completely metabolized in humans. PGE2 is extensively metabolized in the lungs, and the resulting metabolites are further metabolized in the liver and kidney. The major route of elimination of the products of PGE2 metabolism is the kidneys. INDICATIONS AND USAGE PREPIDIL Gel is indicated for ripening an unfavorable cervix in pregnant women at or near term with a medical or obstetrical need for labor induction. CONTRAINDICATIONS Endocervically administered PREPIDIL Gel is not recommended for the following:
WARNINGS FOR HOSPITAL USE ONLY Dinoprostone, as with other potent oxytocic agents, should be used only with strict adherence to recommended dosages. Dinoprostone should be administered by physicians in a hospital that can provide immediate intensive care and acute surgical facilities. Women aged 35 years or older, those with complications during pregnancy and those with a gestational age over 40 weeks have been shown to have an increased risk of post-partum disseminated intravascular coagulation. In addition, these factors may further increase the risk associated with labor induction (see ADVERSE REACTIONS, Post-marketing surveillance). Therefore, in these women, use of dinoprostone should be undertaken with caution. Measures should be applied to detect as soon as possible an evolving fibrinolysis in the immediate post-partum phase. The Clinician should be alert that the intracervical placement of dinoprostone gel may result in inadvertent disruption and subsequent embolization of antigenic tissue causing in rare circumstances the development of Anaphylactoid Syndrome of Pregnancy (Amniotic Fluid Embolism) . PRECAUTIONS 1. General Precautions During use, uterine activity, fetal status, and character of the cervix (dilation and effacement) should be carefully monitored either by auscultation or electronic fetal monitoring to detect possible evidence of undesired responses, e.g., hypertonus, sustained uterine contractility, or fetal distress. In cases where there is a history of hypertonic uterine contractility or tetanic uterine contractions, it is recommended that uterine activity and the state of the fetus should be continuously monitored. The possibility of uterine rupture should be borne in mind when high-tone myometrial contractions are sustained. Feto-pelvic relationships should be carefully evaluated before use of PREPIDIL Gel (see CONTRAINDICATIONS). Caution should be exercised in administration of PREPIDIL Gel in patients with:
Caution should be taken so as not to administer PREPIDIL Gel above the level of the internal os. Careful vaginal examination will reveal the degree of effacement which will regulate the size of the shielded endocervical catheter to be used. That is, the 20 mm endocervical catheter should be used if no effacement is present, and the 10 mm catheter should be used if the cervix is 50% effaced. Placement of PREPIDIL Gel into the extra-amniotic space has been associated with uterine hyperstimulation. As PREPIDIL Gel is extensively metabolized in the lung, liver, and kidney, and the major route of elimination is the kidney, PREPIDIL Gel should be used with caution in patients with renal and hepatic dysfunction. 2. Patients With Ruptured Membranes Caution should be exercised in the administration of PREPIDIL Gel in patients with ruptured membranes. The safety of use of PREPIDIL Gel in these patients has not been determined. 3. Drug Interactions PREPIDIL Gel may augment the activity of other oxytocic agents and their concomitant use is not recommended. For the sequential use of oxytocin following PREPIDIL Gel administration, a dosing interval of 6–12 hours is recommended. 4. Carcinogenesis, Mutagenesis, Impairment of Fertility Carcinogenic bioassay studies have not been conducted in animals with PREPIDIL Gel due to the limited indications for use and short duration of administration. No evidence of mutagenicity was observed in the Micronucleus Test or Ames Assay. 5. Pregnancy Teratogenic Effects PREGNANCY CATEGORY C Prostaglandin E2 produced an increase in skeletal anomalies in rats and rabbits. No effect would be expected clinically, when used as indicated, since PREPIDIL Gel is administered after the period of organogenesis. PREPIDIL Gel has been shown to be embryotoxic in rats and rabbits, and any dose that produces sustained increased uterine tone could put the embryo or fetus at risk. See statements under General Precautions. 6. Pediatric Use Safety and effectiveness in pediatric patients have not been established. ADVERSE REACTIONS PREPIDIL Gel is generally well-tolerated. In controlled trials, in which 1731 women were entered, the following events were reported at an occurrence of ≥ 1%:
In addition, in other trials amnionitis and intrauterine fetal sepsis have been associated with extra-amniotic intrauterine administration of PGE2. Uterine rupture has been reported in association with the use of PREPIDIL Gel intracervically. Additional events reported in the literature, associated by the authors with the use of PREPIDIL Gel, included premature rupture of membranes, fetal depression (1 min Apgar < 7), and fetal acidosis (umbilical artery pH < 7.15). Post-marketing surveillance Blood and lymphatic system disorders An increased risk of post-partum disseminated intravascular coagulation has been described in patients whose labor was induced by pharmacological means, either with dinoprostone or oxytocin (see section WARNINGS). The frequency of this adverse event, however, appears to be rare (<1 per 1,000 labors). DRUG ABUSE AND DEPENDENCE No drug abuse or drug dependence has been seen with the use of PREPIDIL Gel. OVERDOSAGE Overdosage with PREPIDIL Gel may be expressed by uterine hypercontractility and uterine hypertonus. Because of the transient nature of PGE2-induced myometrial hyperstimulation, nonspecific, conservative management was found to be effective in the vast majority of the cases; i.e., maternal position change and administration of oxygen to the mother. β-adrenergic drugs may be used as a treatment of hyperstimulation following the administration of PGE2 for cervical ripening. DOSAGE AND ADMINISTRATION NOTE: USE CAUTION IN HANDLING THIS PRODUCT TO PREVENT CONTACT WITH SKIN. WASH HANDS THOROUGHLY WITH SOAP AND WATER AFTER ADMINISTRATION. PREPIDIL Gel should be brought to room temperature (59° to 86°F; 15° to 30°C) just prior to administration. Do not force the warming process by using a water bath or other source of external heat (eg, microwave oven). To prepare the product for use remove the protective end cap (to serve as plunger extension) and insert the protective end cap into the plunger stopper assembly in the barrel of syringe. Choose the appropriate length shielded catheter (10 mm or 20 mm) and aseptically remove the sterile shielded catheter from the package. Careful vaginal examination will reveal the degree of effacement which will regulate the size of the shielded endocervical catheter to be used. That is, the 20 mm endocervical catheter should be used if no effacement is present, and the 10 mm catheter should be used if the cervix is 50% effaced. Firmly attach the catheter hub to the syringe tip as evidenced by a distinct click. Fill the catheter with sterile gel by pushing the plunger assembly to expel air from the catheter prior to administration to the patient. Proper assembly of the dosing apparatus is shown below. 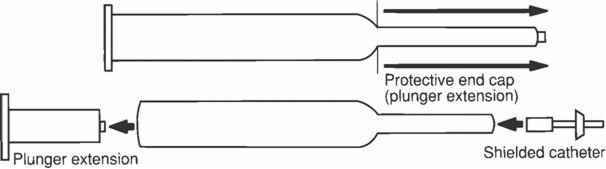 To properly administer the product, the patient should be in a dorsal position with the cervix visualized using a speculum. Using sterile technique, introduce the gel with the catheter provided into the cervical canal just below the level of the internal os. Administer the contents of the syringe by gentle expulsion and then remove the catheter. The gel is easily extrudable from the syringe. Use the contents of one syringe for one patient only. No attempt should be made to administer the small amount of gel remaining in the catheter. The syringe, catheter, and any unused package contents should be discarded after use. Following administration of PREPIDIL Gel, the patient should remain in the supine position for at least 15–30 minutes to minimize leakage from the cervical canal. If the desired response is obtained from PREPIDIL Gel, the recommended interval before giving intravenous oxytocin is 6–12 hours. If there is no cervical/uterine response to the initial dose of PREPIDIL Gel, repeat dosing may be given. The recommended repeat dose is 0.5 mg dinoprostone with a dosing interval of 6 hours. The need for additional dosing and the interval must be determined by the attending physician based on the course of clinical events. The maximum recommended cumulative dose for a 24-hour period is 1.5 mg of dinoprostone (7.5 mL PREPIDIL Gel). HOW SUPPLIED PREPIDIL Gel is available as a sterile semitranslucent viscous preparation for endocervical application: 0.5 mg PGE2 per 3.0 g (2.5 mL) in syringe. In addition, each package contains two shielded catheters (10 mm and 20 mm tip) enclosed in sterile envelopes. The contents are not guaranteed sterile if envelopes are not intact. Each 3 gram syringe applicator contains: 5 × 3 gram syringes NDC 0009-3359-02 PREPIDIL Gel needs to be stored under continuous refrigeration (36° to 46°F; 2° to 8°C). Rx only
July 2008 LAB-0062-4.0 PRINCIPAL DISPLAY PANEL - Package Label NDC 0009-3359-02 Contains 5 of NDC 0009-3359-01 Prepidil® Gel 0.5 mg | |||||||||||||||||||||||||||||||||||||||||||||
普比迪(地诺前列酮)凝胶Prepidil(Dinoprostone Gel)简介:
英文药名: Prepidil(Dinoprostone Gel)
中文药名: 普比迪(地诺前列酮)凝胶
生产品牌药厂家: AstraZeneca
药品介绍
局部使用前列腺素E2凝胶(地诺前列酮)广泛用于促宫颈成熟。使用前列腺素E2凝胶 ... 关键字:普比迪(地诺前列酮)凝胶
责任编辑:admin
|
最新文章更多推荐文章更多热点文章更多
|



