|
【别名】 诺消灵、二羟蒽二酮、二羟基蒽醌 MitoXANTRONE Injection USP Rx ONLY DESCRIPTIONMitoxantrone hydrochloride is a synthetic antineoplastic anthracenedione for intravenous use. The molecular formula is C22H28N4O6•2HCI and the molecular weight is 517.40. It is supplied as a concentrate that MUST BE DILUTED PRIOR TO INJECTION. The concentrate is a sterile, nonpyrogenic, dark blue aqueous solution containing mitoxantrone hydrochloride equivalent to 2 mg/mL mitoxantrone free base, with sodium chloride (0.80% w/v), sodium acetate (0.005% w/v), glacial acetic acid (0.046% w/v), and water for injection as inactive ingredients. The solution has a pH of 3.0 to 4.5 and contains 0.14 mEq of sodium per mL. The product does not contain preservatives. The chemical name is 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]anthraquinone dihydrochloride and the structural formula is: 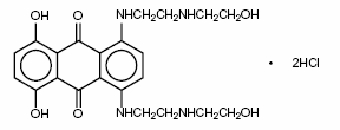 CLINICAL PHARMACOLOGYMechanism of ActionMitoxantrone, a DNA-reactive agent that intercalates into deoxyribonucleic acid (DNA) through hydrogen bonding, causes crosslinks and strand breaks. Mitoxantrone also interferes with ribonucleic acid (RNA) and is a potent inhibitor of topoisomerase II, an enzyme responsible for uncoiling and repairing damaged DNA. It has a cytocidal effect on both proliferating and nonproliferating cultured human cells, suggesting lack of cell cycle phase specificity. Mitoxantrone has been shown in vitro to inhibit B cell, T cell, and macrophage proliferation and impair antigen presentation, as well as the secretion of interferon gamma, TNFα, and IL-2. PharmacokineticsPharmacokinetics of mitoxantrone in patients following a single intravenous administration of mitoxantrone can be characterized by a three-compartment model. The mean alpha half-life of mitoxantrone is 6 to 12 minutes, the mean beta half-life is 1.1 to 3.1 hours and the mean gamma (terminal or elimination) half-life is 23 to 215 hours (median approximately 75 hours). Pharmacokinetic studies have not been performed in humans receiving multiple daily dosing. Distribution to tissues is extensive: steady-state volume of distribution exceeds 1,000 L/m2. Tissue concentrations of mitoxantrone appear to exceed those in the blood during the terminal elimination phase. In the healthy monkey, distribution to brain, spinal cord, eye, and spinal fluid is low. In patients administered 15 to 90 mg/m2 of mitoxantrone intravenously, there is a linear relationship between dose and the area under the concentration-time curve (AUC). Mitoxantrone is 78% bound to plasma proteins in the observed concentration range of 26 to 455 ng/mL. This binding is independent of concentration and is not affected by the presence of phenytoin, doxorubicin, methotrexate, prednisone, prednisolone, heparin, or aspirin. Metabolism and EliminationMitoxantrone is excreted in urine and feces as either unchanged drug or as inactive metabolites. In human studies, 11% and 25% of the dose were recovered in urine and feces, respectively, as either parent drug or metabolite during the 5-day period following drug administration. Of the material recovered in urine, 65% was unchanged drug. The remaining 35% was composed of monocarboxylic and dicarboxylic acid derivatives and their glucuronide conjugates. The pathways leading to the metabolism of mitoxantrone have not been elucidated. Special PopulationsGender -The effect of gender on mitoxantrone pharmacokinetics is unknown. Geriatric -In elderly patients with breast cancer, the systemic mitoxantrone clearance was 21.3 L/hr/m2, compared with 28.3 L/hr/m2 and 16.2 L/hr/m2 for non-elderly patients with nasopharyngeal carcinoma and malignant lymphoma, respectively. Pediatric - Mitoxantrone pharmacokinetics in the pediatric population are unknown. Race -The effect of race on mitoxantrone pharmacokinetics is unknown. Renal Impairment - Mitoxantrone pharmacokinetics in patients with renal impairment are unknown. Hepatic Impairment - Mitoxantrone clearance is reduced by hepatic impairment. Patients with severe hepatic dysfunction (bilirubin > 3.4 mg/dL) have an AUC more than three times greater than that of patients with normal hepatic function receiving the same dose. Patients with hepatic impairment should be treated with caution and dosage adjustment may be required. Drug InteractionsIn vitro drug interaction studies have demonstrated that mitoxantrone did not inhibit CYP450, 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4 across a broad concentration range. The results of in vitro induction studies are inconclusive, but suggest that mitoxantrone may be a weak inducer of CYP450 2E1 activity. Pharmacokinetic studies of the interaction of mitoxantrone with concomitantly administered medications in humans have not been performed. The pathways leading to the metabolism of mitoxantrone have not been elucidated. To date, post-marketing experience has not revealed any significant drug interactions in patients who have received mitoxantrone for treatment of cancer. CLINICAL TRIALSINDICATIONS AND USAGEMitoxantrone in combination with corticosteroids is indicated as initial chemotherapy for the treatment of patients with pain related to advanced hormone-refractory prostate cancer. Mitoxantrone in combination with other approved drug(s) is indicated in the initial therapy of acute nonlymphocytic leukemia (ANLL) in adults. This category includes myelogenous, promyelocytic, monocytic, and erythroid acute leukemias. CONTRAINDICATIONSMitoxantrone is contraindicated in patients who have demonstrated prior hypersensitivity to it. WARNINGSWHEN MITOXANTRONE IS USED IN HIGH DOSES (> 14 mg/m2/d x 3 days) SUCH AS INDICATED FOR THE TREATMENT OF LEUKEMIA, SEVERE MYELOSUPPRESSION WILL OCCUR. THEREFORE, IT IS RECOMMENDED THAT MITOXANTRONE BE ADMINISTERED ONLY BY PHYSICIANS EXPERIENCED IN THE CHEMOTHERAPY OF THIS DISEASE. LABORATORY AND SUPPORTIVE SERVICES MUST BE AVAILABLE FOR HEMATOLOGIC AND CHEMISTRY MONITORING AND ADJUNCTIVE THERAPIES, INCLUDING ANTIBIOTICS. BLOOD AND BLOOD PRODUCTS MUST BE AVAILABLE TO SUPPORT PATIENTS DURING THE EXPECTED PERIOD OF MEDULLARY HYPOPLASIA AND SEVERE MYELOSUPPRESSION. PARTICULAR CARE SHOULD BE GIVEN TO ASSURING FULL HEMATOLOGIC RECOVERY BEFORE UNDERTAKING CONSOLIDATION THERAPY (IF THIS TREATMENT IS USED) AND PATIENTS SHOULD BE MONITORED CLOSELY DURING THIS PHASE. MITOXANTRONE ADMINISTERED AT ANY DOSE CAN CAUSE MYELOSUPPRESSION. GeneralPatients with preexisting myelosuppression as the result of prior drug therapy should not receive mitoxantrone unless it is felt that the possible benefit from such treatment warrants the risk of further medullary suppression. The safety of mitoxantrone injection in patients with hepatic insufficiency is not established (see CLINICAL PHARMACOLOGY). Safety for use by routes other than intravenous administration has not been established. Mitoxantrone is not indicated for subcutaneous, intramuscular, or intra-arterial injection. There have been reports of local/regional neuropathy, some irreversible, following intra-arterial injection. Mitoxantrone must not be given by intrathecal injection. There have been reports of neuropathy and neurotoxicity, both central and peripheral, following intrathecal injection. These reports have included seizures leading to coma and severe neurologic sequelae, and paralysis with bowel and bladder dysfunction. Topoisomerase II inhibitors, including mitoxantrone, have been associated with the development of acute leukemia and myclodysplasia. Cardiac EffectsBecause of the possible danger of cardiac effects in patients previously treated with daunorubicin or doxorubicin, the benefit-to-risk ratio of mitoxantrone therapy in such patients should be determined before starting therapy. Functional cardiac changes including decreases in left ventricular ejection fraction (LVEF) and irreversible congestive heart failure can occur with mitoxantrone. Cardiac toxicity may be more common in patients with prior treatment with anthracyclines, prior mediastinal radiotherapy, or with preexisting cardiovascular disease. Such patients should have regular cardiac monitoring of LVEF from the initiation of therapy. Cancer patients who received cumulative doses of 140 mg/m2 either alone or in combination with other chemotherapeutic agents had a cumulative 2.6% probability of clinical congestive heart failure. In comparative oncology trials, the overall cumulative probability rate of moderate or severe decreases in LVEF at this dose was 13%. Leukemia - Acute congestive heart failure may occasionally occur in patients treated with mitoxantrone for ANLL. In first-line comparative trials of mitoxantrone + cytarabine vs daunorubicin + cytarabine in adult patients with previously untreated ANLL, therapy was associated with congestive heart failure in 6.5% of patients on each arm. A causal relationship between drug therapy and cardiac effects is difficult to establish in this setting since myocardial function is frequently depressed by the anemia, fever and infection, and hemorrhage that often accompany the underlying disease. Hormone-Refractory Prostate Cancer - Functional cardiac changes such as decreases in LVEF and congestive heart failure may occur in patients with hormone-refractory prostate cancer treated with mitoxantrone. In a randomized comparative trial of mitoxantrone plus low-dose prednisone vs low-dose prednisone, 7 of 128 patients (5.5%) treated with mitoxantrone had a cardiac event defined as any decrease in LVEF below the normal range, congestive heart failure (n = 3), or myocardial ischemia. Two patients had a prior history of cardiac disease. The total mitoxantrone dose administered to patients with cardiac effects ranged from > 48 to 212 mg/m2. Among 112 patients evaluable for safety on the mitoxantrone + hydrocortisone arm of the CALGB trial, 18 patients (19%) had a reduction in cardiac function, 5 patients (5%) had cardiac ischemia, and 2 patients (2%) experienced pulmonary edema. The range of total mitoxantrone doses administered to these patients is not available. PregnancyMitoxantrone may cause fetal harm when administered to a pregnant woman. Women of childbearing potential should be advised to avoid becoming pregnant. Mitoxantrone is considered a potential human teratogen because of its mechanism of action and the developmental effects demonstrated by related agents. Treatment of pregnant rats during the organogenesis period of gestation was associated with fetal growth retardation at doses ≥0.1 mg/kg/day (0.01 times the recommended human dose on a mg/m2 basis).When pregnant rabbits were treated during organogenesis, an increased incidence of premature delivery was observed at doses ≥0.1 mg/kg/day (0.01 times the recommended human dose on a mg/m2 basis). No teratogenic effects were observed in these studies, but the maximum doses tested were well below the recommended human dose (0.02 and 0.05 times in rats and rabbits, respectively, on a mg/m2 basis). There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or it the patient becomes pregnant while taking this drug, the patient should be apprised of the potential risk to the fetus. Secondary LeukemiaSecondary leukemia has been reported in cancer patients treated with mitoxantrone. The largest published report involved 1774 patients with breast cancer treated with mitoxantrone in combination with methotrexate with or without mitomycin. In this study, the cumulative probability of developing secondary leukemia was estimated to be 1.1% and 1.6% at 5 and 10 years, respectively. The second largest report involved 449 patients with breast cancer treated with mitoxantrone, usually in combination with radiotherapy and/or other cytotoxic agents. In this study, the cumulative probability of developing secondary leukemia was estimated to be 2.2% at 4 years. Secondary AML has also been reported in cancer patients treated with anthracyclines. Mitoxantrone is an anthracenedione, a related drug. The occurrence of refractory secondary leukemia is more common when anthracyclines are given in combination with DNA-damaging antineoplastic agents, when patients have been heavily pretreated with cytotoxic drugs, or when doses of anthracyclines have been escalated. PRECAUTIONSGeneralTherapy with mitoxantrone should be accompanied by close and frequent monitoring of hematologic and chemical laboratory parameters, as well as frequent patient observation. Systemic infections should be treated concomitantly with or just prior to commencing therapy with mitoxantrone. Information for PatientsMitoxantrone may impart a blue-green color to the urine for 24 hours after administration, and patients should be advised to expect this during therapy. Bluish discoloration of the sclera may also occur. Patients should be advised of the signs and symptoms of myelosuppression. Laboratory TestsA complete blood count, including platelets, should be obtained prior to each course of mitoxantrone and in the event that signs and symptoms of infection develop. Liver function tests should also be performed prior to each course of therapy. In leukemia treatment, hyperuricemia may occur as a result of rapid lysis of tumor cells by mitoxantrone. Serum uric acid levels should be monitored and hypouricemic therapy instituted prior to the initiation of antileukemic therapy. Carcinogenesis, Mutagenesis, Impairment of FertilityCarcinogenesis - Intravenous treatment of rats and mice, once every 21 days for 24 months, with mitoxantrone resulted in an increased incidence of fibroma and external auditory canal tumors in rats at a dose of 0.03 mg/kg (0.02 fold the recommended human dose, on a mg/m2 basis), and hepatocellular adenoma in male mice at a dose of 0.1 mg/kg (0.03 fold the recommended human dose, on a mg/m2 basis). Intravenous treatment of rats, once every 21 days for 12 months with mitoxantrone resulted in an increased incidence of external auditory canal tumors in rats at a dose of 0.3 mg/kg (0.15 fold the recommended human dose, on a mg/m2 basis). Mutagenesis - Mitoxantrone was clastogenic in the in vivo rat bone marrow assay. Mitoxantrone was also clastogenic in two in vitro assays, it induced DNA damage in primary rat heptocytes and sister chromatid exchanges in Chinese hamster ovary cells. Mitoxantrone was mutagenic in bacterial and mammalian test systems (Ames/Salmonella and E. coli and L5176Y TK+/-mouse lymphoma). Drug InteractionsMitoxantrone and its metabolites are excreted in bile and urine, but it is not known whether the metabolic or excretory pathways are saturable, may be inhibited or induced, or if mitoxantrone and its metabolites undergo enterohepatic circulation. To date, post-marketing experience has not revealed any significant drug interactions in patients who have received mitoxantrone for treatment of cancer. Following concurrent administration of mitoxantrone with corticosteroids, no evidence of drug interactions has been observed. Special PopulationsHepatic Impairment - Mitoxantrone should be administered with caution to patients with hepatic impairment. In patients with severe hepatic impairment, the AUC is more than three times greater than the value observed in patients with normal hepatic function. PregnancyNursing MothersMitoxantrone is excreted in human milk and significant concentrations (18 ng/mL) have been reported for 28 days after the last administration. Because of the potential for serious adverse reactions in infants from mitoxantrone, breast feeding should be discontinued before starting treatment. Pediatric UseSafety and effectiveness in pediatric patients have not been established. Geriatric UseHormone-Refractory Prostate Cancer - One hundred forty-six patients aged 65 and over and 52 younger patients (<65 years) have been treated with mitoxantrone in controlled clinical studies. These studies did not include sufficient numbers of younger patients to determine whether they respond differently from older patients. However, greater sensitivity of some older individuals cannot be ruled out. Acute Nonlymphocytic Leukemia - Although definitive studies with mitoxantrone have not been performed in geriatric patients with ANLL, toxicity may be more frequent in the elderly. Elderly patients are more likely to have age-related comorbidities due to disease or disease therapy. ADVERSE REACTIONSLeukemiaMitoxantrone has been studied in approximately 600 patients with ANLL. Table 2 represents the adverse reaction experience in the large U.S. comparative study of mitoxantrone + cytarabine vs daunorubicin + cytarabine. Experience in the large international study was similar. A much wider experience in a variety of other tumor types revealed no additional important reactions other than cardiomyopathy (see WARNINGS). It should be appreciated that the uled adverse reaction categories include overlapping clinical symptoms related to the same condition, e.g., dyspnea, cough and pneumonia. In addition, the uled adverse reactions cannot all necessarily be attributed to chemotherapy as it is often impossible to distinguish effects of the drug and effects of the underlying disease. It is clear, however, that the combination of mitoxantrone + cytarabine was responsible for nausea and vomiting, alopecia, mucositis/stomatitis, and myelosuppression. Table 2 summarizes adverse reactions occurring in patients treated with mitoxantrone + cytarabine in comparison with those who received daunorubicin + cytarabine for therapy of ANLL in a large multicenter randomized prospective U.S. trial. Adverse reactions are presented as major categories and selected examples of clinically significant subcategories.
Hormone-Refractory Prostate CancerDetailed safety information is available for a total of 353 patients with hormone-refractory prostate cancer treated with mitoxantrone, including 274 patients who received mitoxantrone in combination with corticosteroids. Table 3 summarizes adverse reactions of all grades occurring in ≥ 5% of patients in Trial CCI-NOV22.
Table 4 summarizes adverse events of all grades occurring in ≥ 5% of patients in Trial CALGB 9182.
GeneralAllergic Reaction - Hypotension, urticaria, dyspnea, and rashes have been reported occasionally. Anaphylaxis/anaphylactoid reactions have been reported rarely. Cutaneous - Extravasation at the infusion site has been reported, which may result in erythema, swelling, pain, burning, and/or blue discoloration of the skin. Extravasation can result in tissue necrosis with resultant need for debridement and skin grafting. Phlebitis has also been reported at the site of the infusion. Hematologic - Topoisomerase II inhibitors, including mitoxantrone, in combination with other antineoplastic agents, have been associated with the development of acute leukemia (see WARNINGS). Leukemia - Myelosuppression is rapid in onset and is consistent with the requirement to produce significant marrow hypoplasia in order to achieve a response in acute leukemia. The incidences of infection and bleeding seen in the U.S. trial are consistent with those reported for other standard induction regimens. Hormone-Refractory Prostate Cancer -In a randomized study where dose escalation was required for neutrophil counts greater than 1000/mm3, Grade 4 neutropenia (ANC < 500 /mm3) was observed in 54% of patients treated with mitoxantrone + low-dose prednisone. In a separate randomized trial where patients were treated with 14 mg/m2, Grade 4 neutropenia in 23% of patients treated with mitoxantrone + hydrocortisone was observed. Neutropenic fever/infection occurred in 11% and 10% of patients receiving mitoxantrone + corticosteroids, respectively, on the two trials. Platelets <50,000/mm3 were noted in 4% and 3% of patients receiving mitoxantrone + corticosteroids on these trials, and there was one patient death on mitoxantrone + hydrocortisone due to intracranial hemorrhage after a fall. Gastrointestinal - Nausea and vomiting occurred acutely in most patients and may have contributed to reports of dehydration, but were generally mild to moderate and could be controlled through the use of antiemetics. Stomatitis/mucositis occurred within 1 week of therapy. Cardiovascular - Congestive heart failure, tachycardia, EKG changes including arrhythmias, chest pain, and asymptomatic decreases in left ventricular ejection fraction have occurred. (See WARNINGS.) Pulmonary - Interstitial pneumonitis has been reported in cancer patients receiving combination chemotherapy that included mitoxantrone. OVERDOSAGEThere is no known specific antidote for mitoxantrone. Accidental overdoses have been reported. Four patients receiving 140 to 180 mg/m2 as a single bolus injection died as a result of severe leukopenia with infection. Hematologic support and antimicrobial therapy may be required during prolonged periods of severe myelosuppression. Although patients with severe renal failure have not been studied, mitoxantrone is extensively tissue bound and it is unlikely that the therapeutic effect or toxicity would be mitigated by peritoneal or hemodialysis. DOSAGE AND ADMINISTRATION(See also WARNINGS.) Hormone-Refractory Prostate Cancer: Based on data from two Phase 3 comparative trials of mitoxantrone plus corticosteroids versus corticosteroids alone, the recommended dosage of mitoxantrone is 12 to 14 mg/m2 given as a short intravenous infusion every 21 days. Combination Initial Therapy for ANLL in Adults: For induction, the recommended dosage is 12 mg/m2 of mitoxantrone daily on Days 1 to 3 given as an intravenous infusion, and 100 mg/m2 of cytarabine for 7 days given as a continuous 24-hour infusion on Days 1 to 7. Most complete remissions will occur following the initial course of induction therapy. In the event of an incomplete antileukemic response, a second induction course may be given. Mitoxantrone should be given for 2 days and cytarabine for 5 days using the same daily dosage levels. If severe or life-threatening nonhematologic toxicity is observed during the first induction course, the second induction course should be withheld until toxicity resolves. Consolidation therapy which was used in two large randomized multicenter trials consisted of mitoxantrone, 12 mg/m2 given by intravenous infusion daily on Days 1 and 2 and cytarabine, 100 mg/m2 for 5 days given as a continuous 24-hour infusion on Days 1 to 5. The first course was given approximately 6 weeks after the final induction course, the second was generally administered 4 weeks after the first. Severe myelosuppression occurred. (See CLINICAL PHARMACOLOGY.) Hepatic Impairment: For patients with hepatic impairment, there is at present no laboratory measurement that allows for dose adjustment recommendations. (See CLINICAL PHARMACOLOGY, Special Populations, Hepatic Impairment.) Preparation and Administration PrecautionsMITOXANTRONE INJECTION MUST BE DILUTED PRIOR TO USE. The dose of mitoxantrone should be diluted to at least 50 mL with either 0.9% Sodium Chloride Injection or 5% Dextrose Injection. Mitoxantrone may be further diluted into Dextrose 5% in Water, Normal Saline or Dextrose 5% with Normal Saline and used immediately. DO NOT FREEZE. Mitoxantrone should not be mixed in the same infusion as heparin since a precipitate may form. Because specific compatibility data are not available, it is recommended that mitoxantrone not be mixed in the same infusion with other drugs. The diluted solution should be introduced lowly into the tubing as a freely running intravenous infusion of 0.9% Sodium Chloride Injection or 5% Dextrose Injection over a period of not less than 3 minutes. Unused infusion solutions should be discarded immediately in an appropriate fashion. In the case of multidose use, after penetration of the stopper, the remaining portion of the undiluted mitoxantrone injection should be stored not longer than 7 days between 15° to 25°C (59° to 77°F) or 14 days under refrigeration. DO NOT FREEZE. CONTAINS NO PRESERVATIVE. Care in the administration of mitoxantrone will reduce the chance of extravasation. Mitoxantrone should be administered into the tubing of a freely running intravenous infusion of sodium chloride injection (0.9%) or 5% dextrose injection. The tubing should be attached to a Butterfly needle or other suitable device and inserted preferably into a large vein. If possible, avoid veins over joints or in extremities with compromised venous or lymphatic drainage. Care should be taken to avoid extravasation at the infusion site and to avoid contact of mitoxantrone with the skin, mucous membranes or eyes. MITOXANTRONE SHOULD NOT BE ADMINISTERED SUBCUTANEOUSLY. If any signs or symptoms of extravasation have occurred, including burning, pain, pruritis, erythema, swelling, blue discoloration, or ulceration, the injection or infusion should be immediately terminated and restarted in another vein. During intravenous administration of mitoxantrone extravasation may occur with or without an accompanying stinging or burning sensation even if blood returns well on aspiration of the infusion needle. If it is known or suspected that subcutaneous extravasation has occurred, it is recommended that intermittent ice packs be placed over the area of extravasation and that the affected extremity be elevated. Because of the progressive nature of extravasation reactions, the area of injection should be frequently examined and surgery consultation obtained early if there is any sign of a local reaction. Skin accidentally exposed to mitoxantrone should be rinsed copiously with warm water and if the eyes are involved, standard irrigation techniques should be used immediately. The use of goggles, gloves, and protective gowns is recommended during preparation and administration of the drug. Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. HOW SUPPLIEDMitoxantrone Injection USP is a sterile aqueous solution containing mitoxantrone hydrochloride at a concentration equivalent to 2 mg mitoxantrone free base per mL supplied in vials for multidose use as follows: NDC 55390-083-01; 10 mL/multidose vial (20 mg); individually-boxed 原产地英文商品名: 原产地英文商品名: 原产地英文商品名: 原产地英文商品名: 原产地英文商品名: 原产地英文商品名: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
盐酸米托蒽醌注射液(MITOXANTRON,商品名:诺肖林Novantrone)简介:
【别名】 诺消灵、二羟蒽二酮、二羟基蒽醌 【英文名】 Mitoxantrone,Novantrone,Dihydroxanthracenedione,DHAD,MA? 【作用特点】 本品为人工合成的蒽环类抗肿瘤药,为仿阿霉素药品,其作用机制类似 ... 责任编辑:admin
|
最新文章更多推荐文章更多热点文章更多 |




