|
英文药名: Gilenya (Fingolimod Hydrochloride) 中文药名: 芬戈莫德胶囊 生产厂家: 诺华制药
GILENYA is a sphingosine 1-phosphate receptor modulator indicated for treatment of patients with relapsing forms of multiple sclerosis (MS) to reduce the frequency of clinical exacerbations and to delay the accumulation of physical disability. (1) DOSAGE AND ADMINISTRATION Recommended dose: 0.5 mg orally once-daily, with or without food (2) First Dose Monitoring (including re-initiation after discontinuation >14 days): Observe all patients for bradycardia for at least 6 hours; monitor pulse and blood pressure hourly. Electrocardiograms (ECGs) prior to dosing and at end of observation period required. Monitor until resolution if heart rate <45 bpm, atrioventricular (AV) block, or if lowest postdose heart rate is at the end of the observation period. (2) Monitor symptomatic bradycardia with ECG until resolved. Continue overnight if intervention is required; repeat first-dose monitoring for second dose. (2) Observe patients overnight if at higher risk of symptomatic bradycardia, heart block, prolonged QTc interval, or if taking drugs with known risk of torsades de pointes. (2, 7) DOSAGE FORMS AND STRENGTHS 0.5 mg hard capsules (3) CONTRAINDICATIONS Recent myocardial infarction, unstable angina, stroke, transient ischemic attack, decompensated heart failure with hospitalization, or Class III/IV heart failure (4) History of Mobitz Type II 2nd degree or 3rd degree AV block or sick sinus syndrome, unless patient has a pacemaker (4) Baseline QTc interval ≥500 msec (4) Treatment with Class Ia or Class III anti-arrhythmic drugs (4) Hypersensitivity to fingolimod or its excipients (4) WARNINGS AND PRECAUTIONS Infections: GILENYA may increase the risk. Obtain a CBC before initiating treatment. Monitor for infection during treatment and for 2 months after discontinuation. Do not start in patients with active infections. (5.2) Progressive multifocal leukoencephalopathy (PML); Withhold GILENYA at the first sign or symptom suggestive of PML. (5.3) Macular edema: Examine the fundus before and 3–4 months after treatment start. Diabetes mellitus and uveitis increase the risk. (5.4) Posterior reversible encephalopathy syndrome (PRES): If suspected, discontinue GILENYA. (5.5) Respiratory effects: Evaluate when clinically indicated. (5.6) Liver injury: Obtain liver enzyme results before initiation. Closely monitor patients with severe hepatic impairment. Discontinue if significant liver injury occurs. (5.7, 8.6, 12.3) Fetal risk: Women of childbearing potential should use effective contraception during and for 2 months after stopping GILENYA. (5.8) Increased blood pressure (BP): Monitor BP during treatment. (5.9) Basal cell carcinoma: suspicious skin lesions should be evaluated. (5.10) ADVERSE REACTIONS Most common adverse reactions (incidence ≥10% and > placebo): Headache, liver transaminase elevation, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in extremity (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Systemic ketoconazole: Monitor during concomitant use. (7, 12.3) Vaccines: Avoid live attenuated vaccines during, and for 2 months after stopping GILENYA treatment. (5.2, 7) See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 2/2016 FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE GILENYA is indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS) to reduce the frequency of clinical exacerbations and to delay the accumulation of physical disability. 2 DOSAGE AND ADMINISTRATION Recommended Dose The recommended dose of GILENYA is 0.5 mg orally once-daily. Fingolimod doses higher than 0.5 mg are associated with a greater incidence of adverse reactions without additional benefit. GILENYA can be taken with or without food. Patients who initiate GILENYA and those who re-initiate treatment after discontinuation for longer than 14 days require first dose monitoring [see Reinitiation of Therapy Following Discontinuation]. First Dose Monitoring Initiation of GILENYA treatment results in a decrease in heart rate [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)]. After the first dose of GILENYA, the heart rate decrease starts within an hour and the Day 1 nadir generally occurs within approximately 6 hours, although the nadir can be observed up to 24 hours after the first dose in some patients. The first dose of GILENYA should be administered in a setting in which resources to appropriately manage symptomatic bradycardia are available. In order to assess patient response to the first dose of fingolimod, observe all patients for 6 hours for signs and symptoms of bradycardia with hourly pulse and blood pressure measurement. Obtain in all patients an electrocardiogram (ECG) prior to dosing, and at the end of the observation period. Additional observation should be instituted until the finding has resolved in the following situations: The heart rate 6 hours postdose is <45 bpm The heart rate 6 hours postdose is at the lowest value postdose (suggesting that the maximum pharmacodynamic effect on the heart may not have occurred) The ECG 6 hours postdose shows new onset second degree or higher atrioventricular (AV) block Should postdose symptomatic bradycardia occur, initiate appropriate management, begin continuous ECG monitoring, and continue observation until the symptoms have resolved. Should a patient require pharmacologic intervention for symptomatic bradycardia, continuous overnight ECG monitoring in a medical facility should be instituted, and the first dose monitoring strategy should be repeated after the second dose of GILENYA. Patients with some preexisting conditions (e.g., ischemic heart disease, history of myocardial infarction, congestive heart failure, history of cardiac arrest, cerebrovascular disease, uncontrolled hypertension, history of symptomatic bradycardia, history of recurrent syncope, severe untreated sleep apnea, AV block, sinoatrial heart block) may poorly tolerate the GILENYA-induced bradycardia, or experience serious rhythm disturbances after the first dose of GILENYA. Prior to treatment with GILENYA, these patients should have a cardiac evaluation by a physician appropriately trained to conduct such evaluation, and, if treated with GILENYA, should be monitored overnight with continuous ECG in a medical facility after the first dose. GILENYA is contraindicated in patients who in the last 6 months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization or Class III/IV heart failure [see Contraindications (4)]. Since initiation of GILENYA treatment results in decreased heart rate and may prolong the QT interval, patients with a prolonged QTc interval (>450 msec males, >470 msec females) before dosing or during 6 hour observation, or at additional risk for QT prolongation (e.g., hypokalemia, hypomagnesemia, congenital long-QT syndrome), or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes (e.g., citalopram, chlorpromazine, haloperidol, methadone, erythromycin) should be monitored overnight with continuous ECG in a medical facility [see Drug Interactions (7)]. Experience with GILENYA is limited in patients receiving concurrent therapy with drugs that slow heart rate or atrioventricular conduction (e.g., beta blockers, heart-rate lowering calcium channel blockers such as diltiazem or verapamil, or digoxin). Because the initiation of GILENYA treatment is also associated with slowing of the heart rate, concomitant use of these drugs during GILENYA initiation may be associated with severe bradycardia or heart block. The possibility to switch to drugs that do not slow the heart rate or atrioventricular conduction should be evaluated by the physician prescribing these drugs before initiating GILENYA. Patients who cannot switch should have overnight continuous ECG monitoring after the first dose [see Drug Interactions (7)]. Clinical data indicate effects of GILENYA on heart rate are maximal after the first dose although milder effects on heart rate may persist for, on average, 2 to 4 weeks after initiation of therapy at which time heart rate generally returns to baseline. Physicians should continue to be alert to patient reports of cardiac symptoms. Reinitiation of Therapy Following Discontinuation If GILENYA therapy is discontinued for more than 14 days, after the first month of treatment, the effects on heart rate and AV conduction may recur on reintroduction of GILENYA treatment and the same precautions (first dose monitoring) as for initial dosing should apply. Within the first 2 weeks of treatment, first dose procedures are recommended after interruption of 1 day or more; during weeks 3 and 4 of treatment first dose procedures are recommended after treatment interruption of more than 7 days. 3 DOSAGE FORMS AND STRENGTHS GILENYA is available as 0.5 mg hard capsules with a white opaque body and bright yellow cap imprinted with “FTY 0.5 mg” on the cap and 2 radial bands imprinted on the capsule body with yellow ink. 4 CONTRAINDICATIONS Patients who in the last 6 months experienced myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization or Class III/IV heart failure History or presence of Mobitz Type II second-degree or third-degree atrioventricular (AV) block or sick sinus syndrome, unless patient has a functioning pacemaker Baseline QTc interval ≥500 msec Treatment with Class Ia or Class III anti-arrhythmic drugs Patients who have had a hypersensitivity reaction to fingolimod or any of the excipients in GILENYA. Observed reactions include rash, urticaria and angioedema upon treatment initiation [see Warnings and Precautions (5.12)]. 5 WARNINGS AND PRECAUTIONS 5.1 Bradyarrhythmia and Atrioventricular Blocks Because of a risk for bradyarrhythmia and atrioventricular (AV) blocks, patients should be monitored during GILENYA treatment initiation [see Dosage and Administration (2)]. Reduction in Heart Rate After the first dose of GILENYA, the heart rate decrease starts within an hour. On Day 1, the maximum decline in heart rate generally occurs within 6 hours and recovers, although not to baseline levels, by 8 to 10 hours postdose. Because of physiological diurnal variation, there is a second period of heart rate decrease within 24 hours after the first dose. In some patients, heart rate decrease during the second period is more pronounced than the decrease observed in the first 6 hours. Heart rates below 40 beats per minute were rarely observed. In controlled clinical trials, adverse reactions of symptomatic bradycardia following the first dose were reported in 0.6% of patients receiving GILENYA 0.5 mg and in 0.1% of patients on placebo. Patients who experienced bradycardia were generally asymptomatic, but some patients experienced hypotension, dizziness, fatigue, palpitations, and/or chest pain that usually resolved within the first 24 hours on treatment. Following the second dose, a further decrease in heart rate may occur when compared to the heart rate prior to the second dose, but this change is of a smaller magnitude than that observed following the first dose. With continued dosing, the heart rate returns to baseline within 1 month of chronic treatment. Atrioventricular Blocks Initiation of GILENYA treatment has resulted in transient AV conduction delays. In controlled clinical trials, first-degree AV block after the first dose occurred in 4.7% of patients receiving GILENYA and 1.6% of patients on placebo. In a study of 697 patients with available 24-hour Holter monitoring data after their first dose (N=351 receiving GILENYA and N=346 on placebo), second-degree AV blocks (Mobitz Types I [Wenckebach] or 2:1 AV blocks) occurred in 4% (N=14) of patients receiving GILENYA and 2% (N=7) of patients on placebo. Of the 14 patients receiving GILENYA, 7 patients had 2:1 AV block (5 patients within the first 6 hours postdose and 2 patients after 6 hours postdose). All second degree AV blocks on placebo were Mobitz Type I and occurred after the first 12 hours postdose. The conduction abnormalities were usually transient and asymptomatic, and resolved within the first 24 hours on treatment, but they occasionally required treatment with atropine or isoproterenol. Postmarketing Experience In the postmarketing setting, third-degree AV block and AV block with junctional escape have been observed during the first-dose 6-hour observation period with GILENYA. Isolated delayed onset events, including transient asystole and unexplained death, have occurred within 24 hours of the first dose. These events were confounded by concomitant medications and/or preexisting disease, and the relationship to GILENYA is uncertain. Cases of syncope were also reported after the first dose of GILENYA. 5.2 Infections Risk of Infections GILENYA causes a dose-dependent reduction in peripheral lymphocyte count to 20%–30% of baseline values because of reversible sequestration of lymphocytes in lymphoid tissues. GILENYA may therefore increase the risk of infections, some serious in nature [see Clinical Pharmacology (12.2)]. Before initiating treatment with GILENYA, a recent CBC (i.e., within 6 months or after discontinuation of prior therapy) should be available. Consider suspending treatment with GILENYA if a patient develops a serious infection, and reassess the benefits and risks prior to reinitiation of therapy. Because the elimination of fingolimod after discontinuation may take up to 2 months, continue monitoring for infections throughout this period. Instruct patients receiving GILENYA to report symptoms of infections to a physician. Patients with active acute or chronic infections should not start treatment until the infection(s) is resolved. In MS placebo-controlled trials, the overall rate of infections (72%) with GILENYA was similar to placebo. However, bronchitis, herpes zoster, influenza, sinusitis, and pneumonia were more common in GILENYA-treated patients. Serious infections occurred at a rate of 2.3% in the GILENYA group versus 1.6% in the placebo group. In the postmarketing setting, serious infections with opportunistic pathogens including viruses (e.g., John Cunningham virus (JCV), herpes simplex viruses 1 and 2, varicella-zoster virus), fungi (e.g., cryptococci), and bacteria (e.g., atypical mycobacteria) have been reported with GILENYA. Patients with symptoms and signs consistent with any of these infections should undergo prompt diagnostic evaluation and appropriate treatment. Herpes Viral Infections In placebo-controlled trials, the rate of herpetic infections was 9% in patients receiving GILENYA 0.5 mg and 7% on placebo. Two patients died of herpetic infections during controlled trials. One death was due to disseminated primary herpes zoster and the other to herpes simplex encephalitis. In both cases, the patients were taking a 1.25 mg dose of fingolimod (higher than the recommended 0.5 mg dose) and had received high-dose corticosteroid therapy to treat suspected MS relapses. Serious, life-threatening events of disseminated varicella zoster and herpes simplex infections, including cases of encephalitis and multiorgan failure, have occurred with GILENYA in the postmarketing setting. One of these events was fatal. Include disseminated herpetic infections in the differential diagnosis of patients who are receiving GILENYA and present with an atypical MS relapse or multiorgan failure. Cases of Kaposi’s sarcoma have been reported in the postmarketing setting. Kaposi’s sarcoma is an angioproliferative disorder that is associated with infection with human herpes virus 8 (HHV-8). Patients with symptoms or signs consistent with Kaposi’s sarcoma should be referred for prompt diagnostic evaluation and management. Cryptococcal infections Cryptococcal infections, including cases of cryptococcal meningitis and disseminated cryptococcal infections, have been reported with GILENYA in the postmarketing setting. Cryptococcal infections have generally occurred after approximately 2 years of GILENYA treatment, but may occur earlier. The relationship between the risk of cryptococcal infection and the duration of treatment is unknown. Patients with symptoms and signs consistent with a cryptococcal infection should undergo prompt diagnostic evaluation and treatment. Prior and Concomitant Treatment with Antineoplastic, Immunosuppressive, or Immune-Modulating Therapies In clinical studies, patients who received GILENYA did not receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of GILENYA with any of these therapies, and also with corticosteroids, would be expected to increase the risk of immunosuppression [see Drug Interactions (7)]. When switching to GILENYA from immune-modulating or immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects. Varicella Zoster Virus Antibody Testing/Vaccination Patients without a healthcare professional confirmed history of chickenpox or without documentation of a full course of vaccination against varicella zoster virus (VZV) should be tested for antibodies to VZV before initiating GILENYA. VZV vaccination of antibody-negative patients is recommended prior to commencing treatment with GILENYA, following which initiation of treatment with GILENYA should be postponed for 1 month to allow the full effect of vaccination to occur. 5.3 Progressive Multifocal Leukoencephalopathy Cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients with MS who received GILENYA in the postmarketing setting. PML is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. PML has occurred in patients who had not been treated previously with natalizumab, which has a known association with PML, and who were also not taking any immunosuppressive or immunomodulatory medications concomitantly. The patients had no other ongoing identified systemic medical conditions resulting in compromised immune system function, although one patient had a history of cancer treated with chemotherapy several years prior to taking Gilenya. The cases have occurred in patients treated with GILENYA for at least 2 years. The relationship between the risk of PML and the duration of treatment is unknown. At the first sign or symptom suggestive of PML, withhold GILENYA and perform an appropriate diagnostic evaluation. MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. 5.4 Macular Edema Fingolimod increases the risk of macular edema. Perform an examination of the fundus including the macula in all patients before starting treatment, again 3–4 months after starting treatment, and again at any time after a patient reports visual disturbances while on GILENYA therapy. A dose-dependent increase in the risk of macular edema occurred in the GILENYA clinical development program. In 2-year, double-blind, placebo-controlled studies in patients with multiple sclerosis, macular edema with or without visual symptoms occurred in 1.5% of patients (11/799) treated with fingolimod 1.25 mg, 0.5% of patients (4/783) treated with GILENYA 0.5 mg and 0.4% of patients (3/773) treated with placebo. Macular edema occurred predominantly during the first 3 to 4 months of therapy. These clinical trials excluded patients with diabetes mellitus, a known risk factor for macular edema (see below Macular Edema in Patients with History of Uveitis or Diabetes Mellitus). Symptoms of macular edema included blurred vision and decreased visual acuity. Routine ophthalmological examination detected macular edema in some patients with no visual symptoms. Macular edema generally partially or completely resolved with or without treatment after drug discontinuation. Some patients had residual visual acuity loss even after resolution of macular edema. Macular edema has also been reported in patients taking GILENYA in the postmarketing setting, usually within the first 6 months of treatment. Continuation of GILENYA in patients who develop macular edema has not been evaluated. A decision on whether or not to discontinue GILENYA therapy should include an assessment of the potential benefits and risks for the individual patient. The risk of recurrence after rechallenge has not been evaluated. Macular Edema in Patients with History of Uveitis or Diabetes Mellitus Patients with a history of uveitis and patients with diabetes mellitus are at increased risk of macular edema during GILENYA therapy. The incidence of macular edema is also increased in MS patients with a history of uveitis. In the combined clinical trial experience with all doses of fingolimod, the rate of macular edema was approximately 20% in MS patients with a history of uveitis versus 0.6% in those without a history of uveitis. GILENYA has not been tested in MS patients with diabetes mellitus. In addition to the examination of the fundus including the macula prior to treatment and at 3–4 months after starting treatment, MS patients with diabetes mellitus or a history of uveitis should have regular follow-up examinations. 5.5 Posterior Reversible Encephalopathy Syndrome There have been rare cases of posterior reversible encephalopathy syndrome (PRES) reported in patients receiving GILENYA. Symptoms reported included sudden onset of severe headache, altered mental status, visual disturbances, and seizure. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, GILENYA should be discontinued. 5.6 Respiratory Effects Dose-dependent reductions in forced expiratory volume over 1 second (FEV1) and diffusion lung capacity for carbon monoxide (DLCO) were observed in patients treated with GILENYA as early as 1 month after treatment initiation. In 2-year placebo-controlled trials, the reduction from baseline in the percent of predicted values for FEV1 at the time of last assessment on drug was 2.8% for GILENYA 0.5 mg and 1.0% for placebo. For DLCO, the reduction from baseline in percent of predicted values at the time of last assessment on drug was 3.3% for GILENYA 0.5 mg and 0.5% for placebo. The changes in FEV1 appear to be reversible after treatment discontinuation. There is insufficient information to determine the reversibility of the decrease of DLCO after drug discontinuation. In MS placebo-controlled trials, dyspnea was reported in 9% of patients receiving GILENYA 0.5 mg and 7% of patients receiving placebo. Several patients discontinued GILENYA because of unexplained dyspnea during the extension (uncontrolled) studies. GILENYA has not been tested in MS patients with compromised respiratory function. Spirometric evaluation of respiratory function and evaluation of DLCO should be performed during therapy with GILENYA if clinically indicated. 5.7 Liver Injury Elevations of liver enzymes may occur in patients receiving GILENYA. Recent (i.e., within last 6 months) transaminase and bilirubin levels should be available before initiation of GILENYA therapy. In 2-year placebo-controlled clinical trials, elevation of liver transaminases to 3-fold the upper limit of normal (ULN) or greater occurred in 14% of patients treated with GILENYA 0.5 mg and 3% of patients on placebo. Elevations 5-fold the ULN or greater occurred in 4.5% of patients on GILENYA and 1% of patients on placebo. The majority of elevations occurred within 6 to 9 months. In clinical trials, GILENYA was discontinued if the elevation exceeded 5 times the ULN. Serum transaminase levels returned to normal within approximately 2 months after discontinuation of GILENYA. Recurrence of liver transaminase elevations occurred with rechallenge in some patients. Cases of liver injury with hepatocellular and/or cholestatic hepatitis have been reported with GILENYA in the postmarketing setting. Liver enzymes and bilirubin should be monitored in patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine. GILENYA should be discontinued if significant liver injury is confirmed. Patients with preexisting liver disease may be at increased risk of developing elevated liver enzymes when taking GILENYA. Because GILENYA exposure is doubled in patients with severe hepatic impairment, these patients should be closely monitored, as the risk of adverse reactions is greater [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. 5.8 Fetal Risk Based on animal studies, GILENYA may cause fetal harm. Because it takes approximately 2 months to eliminate GILENYA from the body, women of childbearing potential should use effective contraception to avoid pregnancy during and for 2 months after stopping GILENYA treatment. 5.9 Increased Blood Pressure In MS controlled clinical trials, patients treated with GILENYA 0.5 mg had an average increase over placebo of approximately 3 mmHg in systolic pressure, and approximately 2 mmHg in diastolic pressure, first detected after approximately 1 month of treatment initiation, and persisting with continued treatment. Hypertension was reported as an adverse reaction in 8% of patients on GILENYA 0.5 mg and in 4% of patients on placebo. Blood pressure should be monitored during treatment with GILENYA. 5.10 Basal Cell Carcinoma Basal cell carcinoma (BCC) is associated with use of GILENYA. In two-year placebo-controlled trials the incidence of BCC was 2% in patients on GILENYA 0.5 mg and 1% in patients on placebo [see Adverse reactions (6)]. Providers and patients are advised to monitor for suspicious skin lesions. If a suspicious skin lesion is observed, it should be promptly evaluated. 5.11 Immune System Effects Following GILENYA Discontinuation Fingolimod remains in the blood and has pharmacodynamic effects, including decreased lymphocyte counts, for up to 2 months following the last dose of GILENYA. Lymphocyte counts generally return to the normal range within 1–2 months of stopping therapy [see Clinical Pharmacology (12.2)]. Because of the continuing pharmacodynamic effects of fingolimod, initiating other drugs during this period warrants the same considerations needed for concomitant administration (e.g., risk of additive immunosuppressant effects) [see Drug Interactions (7)]. 5.12 Hypersensitivity Reactions Hypersensitivity reactions, including rash, urticaria, and angioedema have been reported with GILENYA in the postmarketing setting. GILENYA is contraindicated in patients with history of hypersensitivity to fingolimod or any of its excipients [see Contraindications (4)]. 6 ADVERSE REACTIONS The following serious adverse reactions are described elsewhere in labeling: Bradyarrhythmia and Atrioventricular Blocks [see Warnings and Precautions (5.1)] Infections [see Warnings and Precautions (5.2)] Progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.3)] Macular Edema [see Warnings and Precautions (5.4)] Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions (5.5)] Respiratory Effects [see Warnings and Precautions (5.6)] Liver Injury [see Warnings and Precautions (5.7)] Fetal Risk [see Warnings and Precautions (5.8)] Increased Blood Pressure [see Warnings and Precautions (5.9)] Basal Cell Carcinoma [see Warnings and Precautions (5.10)] Immune System Effects Following GILENYA Discontinuation [see Warnings and Precautions (5.11)] Hypersensitivity Reactions [see Warnings and Precautions (5.12)] 6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In clinical trials (Studies 1, 2, and 3), a total of 1212 patients with relapsing forms of multiple sclerosis received GILENYA 0.5 mg. This included 783 patients who received GILENYA 0.5 mg in the 2-year placebo-controlled trials (Studies 1 and 3) and 429 patients who received GILENYA 0.5 mg in the 1 year active-controlled trial (Study 2). The overall exposure in the controlled trials was equivalent to 1716 person-years. Approximately 1000 patients received at least 2 years of treatment with GILENYA 0.5 mg. In all clinical studies, including uncontrolled extension studies, the exposure to GILENYA 0.5 mg was approximately 4119 person-years. In placebo-controlled trials, the most frequent adverse reactions (incidence ≥10% and >placebo) for GILENYA 0.5 mg were headache, liver transaminase elevation, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in extremity. Adverse events that led to treatment discontinuation and occurred in more than 1% of patients taking GILENYA 0.5 mg were serum transaminase elevations (4.7% compared to 1% on placebo) and basal cell carcinoma (1% compared to 0.5% on placebo). Table 1 lists adverse reactions that occurred in ≥ 1% of GILENYA-treated patients and ≥ 1% higher rate than for placebo. Table 1 Adverse Reactions Reported in Studies 1 and 3 (Occurring in ≥1% of Patients and Reported for GILENYA 0.5 mg at ≥1% Higher Rate than for Placebo)
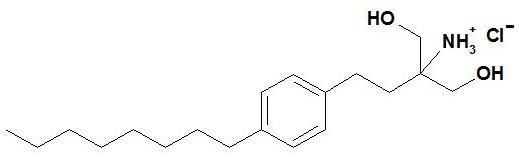
Adverse reactions with GILENYA 0.5 mg in Study 2, the 1-year active-controlled (versus interferon beta-1a) study were generally similar to those in Studies 1 and 3. Vascular Events Vascular events, including ischemic and hemorrhagic strokes, and peripheral arterial occlusive disease were reported in premarketing clinical trials in patients who received GILENYA doses (1.25-5 mg) higher than recommended for use in MS. Similar events have been reported with GILENYA in the postmarketing setting although a causal relationship has not been established. Lymphoma Cases of lymphoma, including both T-cell and B-cell types and CNS lymphoma, have occurred in patients receiving GILENYA. The reporting rate of non-Hodgkin lymphoma with GILENYA is greater than that expected in the general population adjusted by age, gender, and region. The relationship of lymphoma to GILENYA remains uncertain. 7 DRUG INTERACTIONS QT Prolonging Drugs GILENYA has not been studied in patients treated with drugs that prolong the QT interval. Drugs that prolong the QT interval have been associated with cases of torsades de pointes in patients with bradycardia. Since initiation of GILENYA treatment results in decreased heart rate and may prolong the QT interval, patients on QT prolonging drugs with a known risk of torsades de pointes (e.g., citalopram, chlorpromazine, haloperidol, methadone, erythromycin) should be monitored overnight with continuous ECG in a medical facility [see Dosage and Administration (2) and Warnings and Precautions (5.1)]. Ketoconazole The blood levels of fingolimod and fingolimod-phosphate are increased by 1.7-fold when used concomitantly with ketoconazole. Patients who use GILENYA and systemic ketoconazole concomitantly should be closely monitored, as the risk of adverse reactions is greater. Vaccines GILENYA reduces the immune response to vaccination. Vaccination may be less effective during and for up to 2 months after discontinuation of treatment with GILENYA [see Clinical Pharmacology (12.2)]. Avoid the use of live attenuated vaccines during and for 2 months after treatment with GILENYA because of the risk of infection. Antineoplastic, Immunosuppressive, or Immune-Modulating Therapies Antineoplastic, immune-modulating, or immunosuppressive therapies, (including corticosteroids) are expected to increase the risk of immunosuppression, and the risk of additive immune system effects must be considered if these therapies are coadministered with GILENYA. When switching from drugs with prolonged immune effects, such as natalizumab, teriflunomide or mitoxantrone, the duration and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects when initiating GILENYA [see Warnings and Precautions (5.2)]. Drugs That Slow Heart Rate or Atrioventricular Conduction (e.g., beta blockers or diltiazem) Experience with GILENYA in patients receiving concurrent therapy with drugs that slow the heart rate or atrioventricular conduction (e.g., beta blockers, digoxin, or heart rate-slowing calcium channel blockers such as diltiazem or verapamil) is limited. Because initiation of GILENYA treatment may result in an additional decrease in heart rate, concomitant use of these drugs during GILENYA initiation may be associated with severe bradycardia or heart block. Seek advice from the prescribing physician regarding the possibility to switch to drugs that do not slow the heart rate or atrioventricular conduction before initiating GILENYA. Patients who cannot switch, should have overnight continuous ECG monitoring after the first dose [see Dosage and Administration (2) and Warnings and Precautions (5.1)]. Laboratory Test Interaction Because GILENYA reduces blood lymphocyte counts via redistribution in secondary lymphoid organs, peripheral blood lymphocyte counts cannot be utilized to evaluate the lymphocyte subset status of a patient treated with GILENYA. A recent CBC should be available before initiating treatment with GILENYA. 8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Pregnancy Category C There are no adequate and well-controlled studies in pregnant women. In oral studies conducted in rats and rabbits, fingolimod demonstrated developmental toxicity, including teratogenicity (rats) and embryolethality, when given to pregnant animals. In rats, the highest no-effect dose was less than the recommended human dose (RHD) of 0.5 mg/day on a body surface area (mg/m2) basis. The most common fetal visceral malformations in rats included persistent truncus arteriosus and ventricular septal defect. The receptor affected by fingolimod (sphingosine 1-phosphate receptor) is known to be involved in vascular formation during embryogenesis. Because it takes approximately 2 months to eliminate fingolimod from the body, potential risks to the fetus may persist after treatment ends [see Warnings and Precautions (5.8, 5.11)]. GILENYA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Pregnancy Registry A pregnancy registry has been established to collect information about the effect of GILENYA use during pregnancy. Physicians are encouraged to enroll pregnant patients, or pregnant women may register themselves in the GILENYA pregnancy registry by calling Quintiles at 1-877-598-7237, sending an email to gpr@quintiles.com or visiting www.gilenyapregnancyregistry.com. Animal Data When fingolimod was orally administered to pregnant rats during the period of organogenesis (0, 0.03, 0.1, and 0.3 mg/kg/day or 0, 1, 3, and 10 mg/kg/day), increased incidences of fetal malformations and embryo-fetal deaths were observed at all but the lowest dose tested (0.03 mg/kg/day), which is less than the RHD on a mg/m2 basis. Oral administration to pregnant rabbits during organogenesis (0, 0.5, 1.5, and 5 mg/kg/day) resulted in increased incidences of embryo-fetal mortality and fetal growth retardation at the mid and high doses. The no-effect dose for these effects in rabbits (0.5 mg/kg/day) is approximately 20 times the RHD on a mg/m2 basis. When fingolimod was orally administered to female rats during pregnancy and lactation (0, 0.05, 0.15, and 0.5 mg/kg/day), pup survival was decreased at all doses and a neurobehavioral (learning) deficit was seen in offspring at the high dose. The low-effect dose of 0.05 mg/kg/day is similar to the RHD on a mg/m2 basis. 8.2 Labor and Delivery The effects of GILENYA on labor and delivery are unknown. 8.3 Nursing Mothers Fingolimod is excreted in the milk of treated rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from GILENYA, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. 8.4 Pediatric Use The safety and effectiveness of GILENYA in pediatric patients with MS below the age of 18 years have not been established. In a study in which fingolimod (0.3, 1.5, or 7.5 mg/kg/day) was orally administered to young rats from weaning through sexual maturity, changes in bone mineral density and persistent neurobehavioral impairment (altered auditory startle) were observed at all doses. Delayed sexual maturation was noted in females at the highest dose tested and in males at all doses. The bone changes observed in fingolimod-treated juvenile rats are consistent with a reported role of S1P in the regulation of bone mineral homeostasis. When fingolimod (0.5 or 5 mg/kg/day) was orally administered to rats from the neonatal period through sexual maturity, a marked decrease in T-cell dependent antibody response was observed at both doses. This effect had not fully recovered by 6-8 weeks after the end of treatment. 8.5 Geriatric Use Clinical MS studies of GILENYA did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. GILENYA should be used with caution in patients aged 65 years and over, reflecting the greater frequency of decreased hepatic, or renal, function and of concomitant disease or other drug therapy. 8.6 Hepatic Impairment Because fingolimod, but not fingolimod-phosphate, exposure is doubled in patients with severe hepatic impairment, patients with severe hepatic impairment should be closely monitored, as the risk of adverse reactions may be greater [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.3)]. No dose adjustment is needed in patients with mild or moderate hepatic impairment. 8.7 Renal Impairment The blood level of some GILENYA metabolites is increased (up to 13-fold) in patients with severe renal impairment [see Clinical Pharmacology (12.3)]. The toxicity of these metabolites has not been fully explored. The blood level of these metabolites has not been assessed in patients with mild or moderate renal impairment. 10 OVERDOSAGE GILENYA can induce bradycardia as well as AV conduction blocks (including complete AV block). The decline in heart rate usually starts within 1 hour of the first dose and is maximal within 6 hours in most patients [see Warnings and Precautions (5.1)]. In case of GILENYA overdosage, observe patients overnight with continuous ECG monitoring in a medical facility, and obtain regular measurements of blood pressure [see Dosage and Administration (2)]. Neither dialysis nor plasma exchange results in removal of fingolimod from the body. 11 DESCRIPTION Fingolimod is a sphingosine 1-phosphate receptor modulator. Chemically, fingolimod is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol hydrochloride. Its structure is shown below:
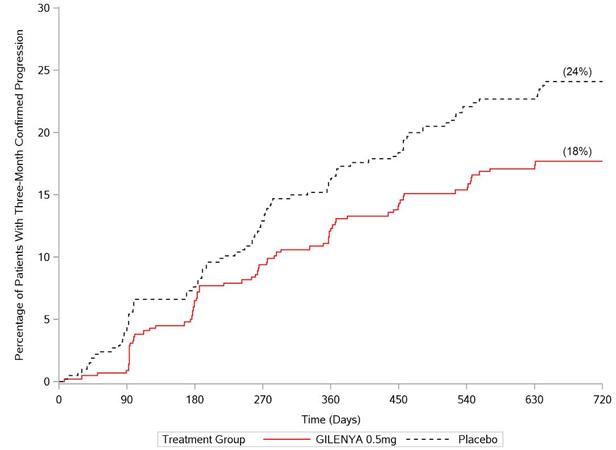
‡Hazard ratio is an estimate of the relative risk of having the event of disability progression on GILENYA as compared to placebo.
Hazard ratio is an estimate of the relative risk of having the event of disability progression on GILENYA as compared to control. Pooled results of study 1 and study 2 showed a consistent and statistically significant reduction of annualized relapse rate compared to comparator in subgroups defined by gender, age, prior MS therapy, and disease activity. 16 HOW SUPPLIED/STORAGE AND HANDLING 0.5 mg GILENYA capsules are hard gelatin capsules with a white opaque body and bright yellow cap imprinted with “FTY 0.5 mg” on the cap and 2 radial bands imprinted on the capsule body with yellow ink. GILENYA capsules are supplied as follows: Bottle of 30 capsules NDC 0078-0607-15 Carton of 7 capsules containing 1 blister card of 7 capsules per blister card NDC 0078-0607-89 GILENYA capsules should be stored at 25ºC (77ºF); excursions permitted to 15ºC–30ºC (59ºF–86ºF). Protect from moisture. 美国包装
|