|
英文药名:Constella(Linaclotide capsules) 中文药名:利那洛肽胶囊 生产厂家:Ironwood制药 Constella 290 micrograms hard capsules
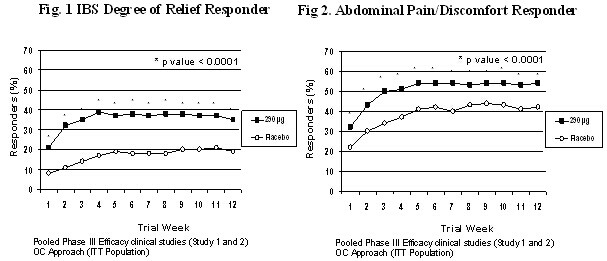
Diarrhoea is the most common adverse reaction and is consistent with the pharmacological action of the active substance. 2% of treated patients experienced severe diarrhoea and 5% of patients discontinued treatment due to diarrhoea in clinical studies. The majority of reported cases of diarrhoea were mild (43%) to moderate (47%); 2% of treated patients experienced severe diarrhoea. Approximately half of the diarrhoea episodes started within the first week of treatment. Regarding duration of diarrhoea, duration of more than 28 days was reported in 21% of patients with diarrhoea; approximately one third of diarrhoea cases resolved within 7 days. Five percent of patients discontinued treatment due to diarrhoea in clinical studies. In those patients in which diarrhoea led to discontinuation, it resolved after a few days of discontinuing treatment. Elderly (>65 years), hypertensive and diabetic patients reported diarrhoea more frequently as compared to the overall IBS-C population included in the clinical trials. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions (see details below). United Kingdom Yellow Card Scheme, Website: www.mhra.gov.uk/yellowcard Ireland IMB Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.imb.ie, e-mail: imbpharmacovigilance@imb.ie 4.9 Overdose An overdose may result in symptoms resulting from an exaggeration of the known pharmacodynamic effects of the medicinal product, mainly diarrhoea. In a study in healthy volunteers receiving a single dose of 2,897 micrograms (up to 10-fold the recommended therapeutic dose) the safety profile in these subjects was consistent with that in the overall population, with diarrhoea being the most commonly reported adverse event. Should an overdose occur, the patient should be treated symptomatically and supportive measures instituted as required. 5. Pharmacological properties 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Other drugs for constipation, ATC Code: A06AX04 Mechanism of action Linaclotide is a Guanylate Cyclase-C receptor agonist (GCCA) with visceral analgesic and secretory activities. Linaclotide is a 14-amino acid synthetic peptide structurally related to the endogenous guanylin peptide family. Both linaclotide and its active metabolite bind to the GC-C receptor, on the luminal surface of the intestinal epithelium. Through its action at GC-C, linaclotide has been shown to reduce visceral pain and increase GI transit in animal models and increase colonic transit in humans. Activation of GC-C results in an increase in concentrations of cyclic guanosine monophosphate (cGMP), both extracellularly and intracellularly. Extracellular cGMP decreases pain-fiber activity, resulting in reduced visceral pain in animal models. Intracellular cGMP causes secretion of chloride and bicarbonate into the intestinal lumen, through activation of the cystic fibrosis transmembrane conductance regulator (CFTR), which results in increased intestinal fluid and accelerated transit. Pharmacodynamic effects In a cross-over food interaction study, 18 healthy subjects were administered Constella 290 micrograms for 7 days both in the fasting and fed state. Taking Constella immediately after a high fat breakfast resulted in more frequent and looser stools, as well as more gastrointestinal adverse events, compared with taking it in the fasted state. Clinical efficacy and safety The efficacy of linaclotide was established in two randomised, double-blind, placebo-controlled Phase 3 clinical studies in patients with IBS-C. In one clinical study (study 1), 802 patients were treated with Constella 290 micrograms or placebo once daily for 26 weeks. In the second clinical study (study 2), 800 patients were treated for 12 weeks, and then re-randomised for an additional 4 weeks treatment period. During the 2-weeks pre-treatment baseline period, patients had a mean abdominal pain score of 5.6 (0-10 scale) with 2.2% of abdominal pain-free days, a mean bloating score of 6.6 (0-10 scale), and an average of 1.8 spontaneous bowel movements (SBM)/week. The characteristics of the patient population included in Phase 3 clinical trials were as follows: mean age of 43.9 years [range 18 - 87 years with 5.3% ≥ 65 years of age], 90.1% female. All patients met Rome II criteria for IBS-C and were required to report a mean abdominal pain score of ≥ 3 on a 0-to-10-point numeric rating scale (criteria that correspond to a moderate to severe IBS population), < 3 complete spontaneous bowel movements and ≤ 5 SBMs per week during a 2-week baseline period. The co-primary endpoints in both clinical studies were 12-week IBS degree of relief responder rate and 12 week abdominal pain/discomfort responder rate. An IBS degree of relief responder was a patient that was considerably or completely relieved for at least 50% of the treatment period; an abdominal pain/discomfort responder was a patient that had an improvement of 30% or more for at least 50% of the treatment period. For the 12 weeks data, study 1 shows that 39% of the patients treated with linaclotide compared with 17% of the patients treated with placebo showed response to IBS degree of relief (p<0.0001) and 54% of the patients treated with linaclotide compared with 39% of the patients treated with placebo showed response to abdominal pain/discomfort (p<0.0001). Study 2 shows that 37% of the patients treated with linaclotide compared with 19% of the patients treated with placebo showed response to IBS degree of relief (p<0.0001) and 55% of the patients treated with linaclotide compared with 42% of the patients treated with placebo showed response to abdominal pain/discomfort (p=0.0002). For the 26 weeks data, study 1 shows that 37% and 54% of the patients treated with linaclotide compared with 17% and 36% of the patients treated with placebo showed response to IBS degree of relief (p<0.0001) and abdominal pain/discomfort (p<0.0001) respectively. In both studies, these improvements were seen by week 1 and sustained over the entire treatment periods (Figures 1 and 2). Linaclotide has been shown not to cause rebound effect when the treatment was stopped after 3 months continuous treatment. Other signs and symptoms of IBS-C including bloating, complete spontaneous bowel movement (CSBM) frequency, straining, stool consistency, were improved in linaclotide treated patients vs. placebo (p<0.0001) as shown in the following table. These effects were reached at 1 week and sustained over the entire treatment periods.
CSBM: Complete Spontaneous Bowel Movement Treatment with linaclotide also resulted in significant improvements in validated and disease-specific Quality of Life measure (IBS-QoL; p<0.0001), and EuroQoL (p = 0.001). Clinically meaningful response in overall IBS-QoL (> 14 points difference) was achieved in 54% of linaclotide treated patients vs. 39% in placebo treated patients. Paediatric population The European Medicines Agency has deferred the obligation to submit the results of clinical studies with Constella in one or more subsets of the paediatric population in functional constipation. See section 4.2 for information on paediatric use. 5.2 Pharmacokinetic properties Absorption In general, linaclotide is minimally detectable in plasma following therapeutic oral doses and therefore standard pharmacokinetic parameters cannot be calculated. Following single doses of up to 966 micrograms and multiple doses up to 290 micrograms of linaclotide, there were no detectable plasma levels of parent compound or the active metabolite (des-tyrosine). When 2,897 micrograms was administered on day 8, following a 7-day course of 290 micrograms/day, linaclotide was detectable in only 2 of 18 subjects at concentrations just above the lower limit of quantification of 0.2 ng/ml (concentrations ranged from 0.212 to 0.735 ng/ml). In the two pivotal phase 3 studies in which patients were dosed with 290 micrograms of linaclotide once daily, linaclotide was only detected in 2 out of 162 patients approximately 2 h following the initial linaclotide dose (concentrations were 0.241 ng/ml to 0.239 ng/ml) and in none of the 162 patients after 4 weeks of treatment. The active metabolite was not detected in any of the 162 patients at any time point. Distribution As linaclotide is rarely detectable in plasma following therapeutic doses, standard distribution studies have not been conducted. It is expected that linaclotide is negligibly or not systemically distributed. Biotransformation Linaclotide is metabolised locally within the gastrointestinal tract to its active primary metabolite, des-tyrosine. Both linaclotide and des-tyrosine active metabolite are reduced and enzymatically proteolyzed within the gastrointestinal tract to smaller peptides and naturally occurring amino acids. The potential inhibitory activity of linaclotide and its active primary metabolite MM-419447 on the human efflux transporters BCRP, MRP2, MRP3, and MRP4 and the human uptake transporters OATP1B1, OATP1B3, OATP2B1, PEPT1 and OCTN1 was investigated in vitro. Results of this study showed that neither peptide is an inhibitor of the common efflux and uptake transporters studied at clinically relevant concentrations. The effect of linaclotide and its metabolites to inhibit the common intestinal enzymes (CYP2C9 and CYP3A4) and liver enzymes (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4) or to induce liver enzymes (CYP1A2, 2B6, and 3A4/5) was investigated in vitro. Results of these studies showed that linaclotide and des-tyrosine metabolite are not inhibitors or inducers of the cytochrome P450 enzyme system. Elimination Following a single oral dose of 2,897 micrograms linaclotide on day 8, after a 7-day course of 290 micrograms/day in 18 healthy volunteers, approximately 3 to 5% of the dose was recovered in the faeces, virtually all of it as the des-tyrosine active metabolite. Age and gender Clinical studies to determine the impact of age and gender on the clinical pharmacokinetics of linaclotide have not been conducted because it is rarely detectable in plasma. Gender is not expected to have any impact on dosing. For age related information, please see sections 4.2., 4.4., and 4.8. Renal impairment Constella has not been studied in patients who have renal impairment. Linaclotide is rarely detectable in plasma, therefore, renal impairment would not be expected to affect clearance of the parent compound or its metabolite. Hepatic impairment Constella has not been studied in patients who have hepatic impairment. Linaclotide is rarely detectable in plasma and is not metabolised by liver cytochrome P450 enzymes, therefore, hepatic impairment would not be expected to affect the metabolism or clearance of the parent drug or its metabolite. 5.3 Preclinical safety data Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential and toxicity to reproduction and development. 6. Pharmaceutical particulars 6.1 List of excipients Capsule contents Cellulose, microcrystalline Hypromellose Calcium chloride dihydrate Leucine Capsule shell Titanium dioxide (E 171) Gelatin Red iron oxide (E172) Yellow iron oxide (E172) Capsule ink Shellac Propylene glycol Ammonia solution, concentrated Potassium hydroxide Titanium dioxide (E 171) Black iron oxide (E172) 6.2 Incompatibilities Not applicable. 6.3 Shelf life Unopened bottle: 3 years. Once the bottle is opened, the capsules should be used within 18 weeks. 6.4 Special precautions for storage Do not store above 30°C. Keep the bottle tightly closed in order to protect from moisture. The bottle contains one or more sealed canisters containing silica gel to keep the capsules dry. Keep the canisters in the bottle. 6.5 Nature and contents of container White, high density polyethylene (HDPE) bottle with a tamper evident seal and a child-resistant screw cap, together with one or more desiccant canisters containing silica gel. Pack sizes: 10, 28, 60 and 90 capsules. Not all pack sizes may be marketed. 6.6 Special precautions for disposal and other handling Any unused medicinal product or waste material should be disposed of in accordance with local requirements. 7. Marketing authorisation holder Almirall, S.A. Ronda General Mitre, 151 08022 Barcelona Spain 8. Marketing authorisation number(s) EU/1/12/801/001 EU/1/12/801/002 EU/1/12/801/003 EU/1/12/801/004 9. Date of first authorisation/renewal of the authorisation Date of first authorisation: 26 November 2012 10. Date of revision of the text 03/2014 Detailed information on this medicinal product is available on the website of the European Medicines Agency http://www.ema.europa.eu. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
利那洛肽胶囊|Constella(Linaclotide capsules)简介:
英文药名:Constella(Linaclotide capsules)
中文药名:利那洛肽胶囊
生产厂家:Ironwood制药药品介绍Almirall, S.A.公司 (ALM:MC)和Ironwood制药公司共同宣布其新药Constella®(利那洛肽胶囊290 ... 责任编辑:admin
|
最新文章更多推荐文章更多热点文章更多 |