REVLIMID(lenalidomide)capsules
 |
授权形式: |
免费版 |
作者/开发商: |
|
| 文件大小: |
33.45 Kb |
解压密码: |
|
| 软件语言: |
简体中文 |
更新时间: |
2013-04-17 |
| 版本号: |
1.0 |
软件平台: |
Win2000/WinXP/Win2003 |
| 软件类别: |
国产软件 |
演示地址: |
|
| 评分等级: |
★★★★★ |
注册地址: |
|
| 发布人: |
admin |
下载次数: |
0(今日:0,本周:0,本月:0) |
| 插件认证: |
无插件 |
浏览次数: |
84 |
REVLIMID® (lenalidomide)
5 mg, 10 mg, 15 mg and 25 mg capsules
REVLIMID is an oral agent approved for use in relapsed or refractory MCL
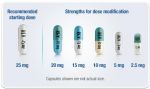
Available in 6 dosage strengths for dosing flexibility
REVLIMID is available in 2.5-mg, 5-mg, 10-mg, 15-mg, 20-mg, and 25-mg capsules. The recommended starting dose for relapsed or refractory MCL is 25 mg/day orally on Days 1-21 of repeated 28-day cycles. Treatment should be continued until disease progression or unacceptable toxicity.
REVLIMID Rx
Add Drug To My List Compare to related Drugs View/edit/Compare drugs in my list
Select the drug indication to add to your list
REVLIMID
Anemias
Leukemias, lymphomas, and other hematologic cancers Only 4 drugs may be compared at once
Generic Name and Formulations:
Lenalidomide 2.5mg, 5mg, 10mg, 15mg, 20mg, 25mg; caps; contains lactose.
Company:
Celgene Corp
Select therapeutic use: Anemias
Leukemias, lymphomas, and other hematologic cancers
Indications for REVLIMID:
Transfusion-dependent anemia due to Low- or Intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality.
Adult:
Swallow whole with water. ≥18yrs: initially 10mg per day; adjust dose based on response. Renal impairment: Moderate (CrCL 30–60mL/min): 5mg per day. Severe (CrCL <30mL/min without dialysis): 2.5mg per day. ESRD (CrCL <30mL/min with dialysis): 2.5mg once daily; administer after dialysis (on dialysis days). Dose adjustments if thrombocytopenia or neutropenia develops: see full labeling.
Children:
<18yrs: not established.
Contraindications:
Pregnancy (Cat. X).
Warnings/Precautions:
Must register patient in Revlimid REMS program; patient must understand toxicity with fetal exposure. Counsel patient on need for contraception; females: use 2 forms of contraception 1 month before, during therapy, during dose interruptions, and 1 month after therapy; males: use condom during and 1 month after therapy; obtain 2 negative pregnancy tests (one within 10–14 days, and then another within 24hrs prior to starting therapy), repeat at least weekly for 1st month then every 4 weeks (regular menstrual cycles) or every 2 weeks (irregular cycles); get informed consent. Do not donate blood during and for 1 month after therapy. Monitor for signs/symptoms of thromboembolic events; base thromboprophylaxis on patient's risks. Obtain CBCs weekly for first 8 weeks, then monthly; dose interruption and/or reduction may be needed. May require blood product support and/or growth factors. Renal impairment (monitor). Monitor for tumor lysis syndrome in those with high tumor burden. Monitor liver enzymes; discontinue if elevation occurs. Lactose intolerance. Maximum 1 month per ℞. Nursing mothers: not recommended.
Interactions:
Monitor digoxin. Concomitant warfarin; monitor PT, INR. May increase risk of thrombosis with dexamethasone, erythropoietic agents, or estrogen containing therapies.
Pharmacological Class:
Immunomodulator.
Adverse Reactions:
Birth defects, thrombocytopenia, neutropenia, anemia, leukopenia, constipation, diarrhea, nausea, vomiting, pruritus, rash, fatigue, arthralgia, pyrexia, back pain, cough, dizziness, headache, dyspnea, nasopharyngitis, epistaxis, upper respiratory tract infection, tremor, blurred vision, muscle cramp, decreased appetite, peripheral edema; thrombosis/embolism, allergic reactions (discontinue if occurs; do not resume), tumor flare reaction (monitor; esp. in treating MCL), hepatotoxicity.
Note:
Available only through Revlimid REMS program. Report any suspected fetal exposure to the FDA at (800) FDA-1088 and Celgene at (888) 423-5436.
REMS:
YES
How Supplied:
Caps 2.5mg, 5mg, 10mg—28, 100; 15mg, 20mg, 25mg—21, 100
https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=10442
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fa97bf5-28a2-48f1-8955-f56012d296be
|


