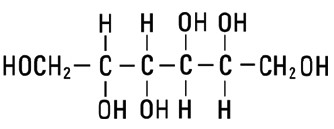
甘露醇Mannitol
中文别名:甘露醇、甘露糖醇、六己醇、水蜜醇
英文别名:Isotol、Manicol、Manita、Manna Sugar、Mannistol、Mannite、Osmitrol、Osmofundin、Osmosal、Resectisol、V-Mannitol
药品类别:脱水药
药理药动
药效学
甘露醇为单糖,在体内不被代谢,经肾小球滤过后在肾小管内甚少被重吸收,起到渗透利尿作用。
(1) 组织脱水作用。提高血浆胶体通透压,导致组织内(包括眼、脑、脑脊液等)水分进入血管内,从而减轻组织水肿,降低眼内压、颅内压和脑脊液容量及其压力。1g甘露醇可产生渗透浓度为5.5mOsm,注射100g甘露醇可使2000ml细胞内水转移至细胞外, 尿钠排泄50g。(2) 利尿作用。甘露醇的利尿作用机制分两个方面。①甘露醇增加血容量,并促进前列腺素I2分泌, 从而扩张肾血管,增加肾血流量包括肾髓质血流量。肾小球入球小动脉扩张, 肾小球毛细血管压升高, 皮质肾小球滤过率升高。②本药自肾小球滤过后极少(<10%)由肾小管重吸收,故可提高肾小管内液渗透浓度,减少肾小管对水及Na+、CI-、K+、Ca2+、Mg2+和其他溶质的重吸收。 过去认为本药主要作用于近端小管,但经穿刺动物实验发现,应用大剂量甘露醇后,通过近端小管的水和Na+仅分别增多10~20%和4~5%;而到达远端小管的水和Na+则分别增加40%和25%。揭示亨氏袢重吸收水和Na+减少在甘露醇利尿作用中占重要地位。此可能是由于肾髓质血流量增加,髓质内尿素和Na+流失增多,从而破坏了髓质渗透压梯度差。
由于输注甘露醇后肾小管液流量增加,当某些药物毒物中毒时,这些物质在肾小管内浓度下降,对肾脏毒性减小,而且经肾脏排泄加快。
药动学
甘露醇口服吸收很少。静脉注射后迅速进入细胞外液而不进入细胞内。利尿作用于静注后0.5~1小时出现, 维持3小时。降低眼内压和颅内压作用于静注后15分钟内出现, 达峰时间为30~60分钟, 维持3~8小时。本药可由肝脏生成糖原,但由于静脉注射后迅速经肾脏排泄, 故一般情况下经肝脏代谢的量很少。本药半衰期为100分钟, 当存在急性肾功能衰竭时可延长至6小时。肾功能正常时,静脉注射甘露醇100g, 3小时内80%经肾脏排出。
适 应 症
(1) 组织脱水药。用于治疗各种原因引起的脑水肿,降低颅内压,防止脑疝。
(2) 降低眼内压。可有效降低眼内压,应用于其他降眼内压药无效时或眼内手术前准备。
(3) 渗透性利尿药。应用于预防各种原因起的急性肾小管球死,并用于鉴别肾前性因素或急性肾功能衰竭引起的少尿。
(4) 作为辅助性利尿措施治疗肾病综合征、肝硬化腹水,尤其是当伴有低蛋白血症时。
(5) 对某些药物逾量或药物中毒(如巴比妥类药物、锂、水杨酸盐和溴化物等),本药可促进上述物质的排泄,并防止肾毒性。
(6) 作为冲洗剂,应用于经尿道内作前列腺切除术。
(7) 术前肠道准备。
用法用量
1.成人常用量
(1)利尿。常用量为按体重 1—2g/kg,一般用 20%溶液250ml静脉滴注,并调整剂量使尿量维持在每小时 30—50ml。
(2)治疗脑水肿、颅内高压和青光眼。按体重 1.5—2g/kg,配制为 15—25%浓度于 20—60分钟内静脉滴注。每日可给 3次。当病人衰弱时,剂量应减小至 0.5g/kg。
(3)鉴别肾前性少尿和肾性少尿。按体重 0.2g/kg,以 20%浓度于 3—5分钟内静脉滴注,如用药后 2~3小时以后每小时尿量仍低于 30—50ml,最多再试用一次,如仍无反应则应停药。已有心功能减退或心力衰竭者慎用或不宜使用。
(4)预防急性肾小管坏死。先给予 12.5—25g,10分钟内静脉滴注,若无特殊情况,再给50g 1小时内静脉滴注,若尿量能维持在每小时 50ml以上,则可继续应用 5%溶液静滴;若无效则立即停药。
(5)治疗药物、毒物中毒。50g以20%溶液静滴,调整剂量使尿量维持在每小时 100—500ml。
(6)肠道准备。术前 4—8小时,10%溶液 1000ml于 30分钟内口服完毕;
2.小儿常用量
(1)利尿。按体重 2g/kg或按体表面积60g/平方米,以 15—20%溶液 2~6小时内静脉滴注。
(2)治疗脑水肿、颅内高压和青光眼。按体重 1—2g/kg或按体表面积 30~60g/平方米,以15—20%浓度溶液于 30—60分钟内静脉滴注。病人衰弱时剂量减至 0.5g/kg。
(3)鉴别肾前性少尿和肾性少尿。按体重 0.2g/kg或按体表面积 6g/平方米,以 15—25%浓度静脉滴注 3—5分钟,如用药后 2—3小时尿量无明显增多,可再用 1次,如仍无反应则不再使用。
(4)治疗药物、毒物中毒。按体重 2g/kg或按体表面积60g/平方米以 5—10%溶液静脉滴注。
[制剂与规格]甘露醇注射液(1)50ml:10g(2)100ml:20g(3)250ml:50g
静脉滴注,按1-4.5g/kg计,用20%溶液250-500ml,滴速10ml/分.不能注入过快。
不良反应
(1)水和电解质紊乱。最为常见。①快速大量静注甘露醇可引起体内甘露醇积聚,血容量迅速大量增多,导致心力衰竭(尤其有心功能损害时),稀释性低钠血症,偶可致高钾血症。②不适当的过度利尿导致血容量减少,加重少尿。
(2)寒战、发热。
(3)排尿困难。
(4)血栓性静脉炎。
(5)甘露醇外渗可致组织水肿、皮肤坏死。
(6)过敏引起皮疹、荨麻疹、呼吸困难、过敏性休克。
(7)头晕、视力模糊。
(8)高渗引起口渴。
(9)渗透性肾病(或称甘露醇肾病),主要见于大剂量快速静脉滴注时。其机理尚未完全阐明,可能与甘露醇引起肾小管液渗透压上升过高,导致肾小管上皮细胞损伤。病理表现为肾小管上皮细胞肿胀,空泡形成临床上出现尿量减少,甚至急性肾功能衰竭。渗透性肾病常见于老年肾血流量减少及低钠、脱水患者。
注入过快可引起头痛,视物模糊,眩晕,畏寒,注射部位疼痛.可有接触性皮炎,皮疹,急性肾衰。对于肾功能不全的病人,会造成低血钠症和结肠内氢离子浓度过高。
本品可以引起皮肤过敏和全身性荨麻疹。主要的不良反应和毒性反应是造成电解质紊乱和急性的肾功能衰竭。
注射过快时有一过性头痛、视力模糊、眩晕、畏寒等,甚至出现神志不清、抽搐。这些可能是中枢神经系统疾病本身表现,也可能由于脱水过多,诱发高渗透压症群的表现,可有硬脑膜下或蛛网膜下出血,需要作出鉴别,测血浆渗透压可有帮助。在剂量较大如8小时内超过200g时。则可出现胸闷、胸痛、心律不齐或过速、咳嗽、呼吸时有哮鸣音或肺基部出现罗音、颈静脉怒张等,这些可由于心功能不全或注射甘露醇过速、用量过多、肾排泄功能不佳,以致心脏负荷过重所引起,严重时可导致急性肺水肿。
甘露醇静脉输入还可有肌力软弱、行动不便、手脚麻木、有刺痛感或肌痉挛等,这可能因甘露醇应用后导致利尿、脱水、失钠从而引起水、电解质紊乱,若患者原来存在失水或血容不足,则症状更易出现。
在注射甘露醇的同时应纠正上述紊乱。持续大剂量应用甘露醇还可引起高渗性肾病(有称甘露醇性肾病),患者尿量减少甚至达少尿程度(每日排尿少于400ml),可出现浮肿,高渗性昏迷等症状,肾穿针活组织检查可发现肾小管上皮细胞肿胀,应即停用,给予葡萄糖注射液或葡萄糖氯化钠注射液,以降低血浆渗透压。
其急性肾衰的发生与使用大剂量甘露醇有关。使用大剂量甘露醇后,在致密斑部位造成一个异常强烈的传入刺激,导致肾单位滤过率明显下降,而发生急性肾衰。
其急性肾衰的发生与使用大剂量甘露醇有关。使用大剂量甘露醇后,在致密斑部位造成一个异常强烈的传入刺激,导致肾单位滤过率明显下降,而发生急性肾衰。
高敏反应的人注射甘露醇后可发生过敏反应,在滴注药物的3~5分钟后出现喷嚏、流鼻涕、舌肿、呼吸困难、意识丧失等,应立即停药,对症处理。
高渗甘露醇注射时可引起静脉炎,局部出现红肿及疼痛,注射处有渗漏时可引起局部皮肤坏死。
本品也可致有所谓的糖贮存肾伴有草酸钙沉淀。
禁忌症
(1)甘露醇能透过胎盘屏障。
(2)是否能经乳汁分泌尚不清楚。
(3)小儿应用本药无特殊注意事项。
(4)老年人应用本药较易出现肾损害,且随年龄增长,发生肾损害的机会增多。
(5)下列情况慎用:①明显心肺功能损害者,因本药所致的突然血容量增多可引起充血性心力衰竭;②高钾血症或低钠血症;③低血容量,应用后可因利尿而加重病情,或使原来低血容量情况被暂时性扩容所掩盖;④严重肾功能不全而排泄减少使本药在体内积聚,引起血容量明显增加,加重心脏负荷,诱发或加重心力衰竭;⑤对甘露醇不能耐受者。
(6)下列情况禁用:①已确诊为急性肾小管坏死的无尿患者,包括对试用甘露醇无反应者,因甘露醇积聚引起血容量增多,加重心脏负担;②严重失水者;③颅内活动性出血者,因扩容加重出血,但颅内手术时除外;④急性肺水肿,或严重肺瘀血。
心功能不全,脱水少尿者慎用。对于肾功能不全的病人,会造成低血钠症和结肠内氢离子浓度过高。
药物相互作用
(1)可增加洋地黄毒性作用,与低钾血症有关。
(2)增加利尿药及碳酸酐酶抑制剂的利尿和降眼内压作用,与这些药物合并时应调整剂量。
Mannitol
Pronunciation: MAN-ih-tole
Generic Name: Mannitol
Brand Name: Osmitrol
Mannitol is used for:
Preventing or treating excess body water in certain kidney conditions, reducing swelling of the brain, or reducing pressure in the eye. It may also be used for other conditions as determined by your doctor.
Mannitol is an osmotic diuretic. It works by increasing the amount of fluid excreted by the kidneys and helps the body to decrease pressure in the brain and eyes.
Do NOT use Mannitol if:
•you are allergic to any ingredient in Mannitol
•you have a history of heart failure
•you have decreased or absent production of urine due to severe kidney disease, certain severe lung problems (eg, pulmonary congestion or pulmonary edema), bleeding in the brain, or severe dehydration
Contact your doctor or health care provider right away if any of these apply to you.
Before using Mannitol:
Some medical conditions may interact with Mannitol. Tell your doctor or pharmacist if you have any medical conditions, especially if any of the following apply to you:
•if you are pregnant, planning to become pregnant, or are breast-feeding
•if you are taking any prescription or nonprescription medicine, herbal preparation, or dietary supplement
•if you have allergies to medicines, foods, or other substances
•if you have swelling, kidney problems, or heart problems (eg, congestive heart failure)
Some MEDICINES MAY INTERACT with Mannitol. However, no specific interactions with Mannitol are known at this time.
Ask your health care provider if Mannitol may interact with other medicines that you take. Check with your health care provider before you start, stop, or change the dose of any medicine.
How to use Mannitol:
Use Mannitol as directed by your doctor. Check the label on the medicine for exact dosing instructions.
•Mannitol is usually administered as an injection at your doctor's office, hospital, or clinic.
•If Mannitol contains particles or is discolored, or if the vial is cracked or damaged in any way, do not use it.
•Keep this product, as well as syringes and needles, out of the reach of children and pets. Do not reuse needles, syringes, or other materials. Ask your health care provider how to dispose of these materials after use. Follow all local rules for disposal.
•If you miss a dose of Mannitol, contact your doctor right away.
Ask your health care provider any questions you may have about how to use Mannitol.
Important safety information:
•Mannitol may cause dizziness. These effects may be worse if you take it with alcohol or certain medicines. Use Mannitol with caution. Do not drive or perform other possibly unsafe tasks until you know how you react to it.
•Tell your doctor immediately if you have difficulty urinating or experience extreme dizziness.
•Lab tests, including blood electrolytes, kidney function, lung function, heart function, and blood counts, may be performed to monitor your progress or to check for side effects. Be sure to keep all doctor and lab appointments.
•Use Mannitol with caution in the ELDERLY; they may be more sensitive to its effects.
•Mannitol should be used with extreme caution in CHILDREN younger than 12 years old; safety and effectiveness in these children have not been confirmed.
•PREGNANCY and BREAST-FEEDING: It is not known if Mannitol can cause harm to the fetus. If you become pregnant, contact your doctor. You will need to discuss the benefits and risks of using Mannitol while you are pregnant. It is not known if Mannitol is found in breast milk. If you are or will be breast-feeding while you use Mannitol, check with your doctor. Discuss any possible risks to your baby.
Possible side effects of Mannitol:
All medicines may cause side effects, but many people have no, or minor, side effects. Check with your doctor if any of these most COMMON side effects persist or become bothersome:
Increased urination; nausea; runny nose; vomiting.
Seek medical attention right away if any of these SEVERE side effects occur:
Severe allergic reactions (rash; hives; itching; difficulty breathing; tightness in the chest; swelling of the mouth, face, lips, or tongue); blurred vision; chest pain; chills or fever; confusion; decreased alertness; difficulty urinating; extreme dizziness; extreme thirst or dry mouth; fast or irregular heartbeat; headache; muscle cramps; pain, redness, or swelling at the injection site; weakness.
This is not a complete list of all side effects that may occur. If you have questions about side effects, contact your health care provider. Call your doctor for medical advice about side effects. To report side effects to the appropriate agency, please read the Guide to Reporting Problems to FDA.
If OVERDOSE is suspected:
Contact 1-800-222-1222 (the American Association of Poison Control Centers), your local poison control center, or emergency room immediately.
Proper storage of Mannitol:
Mannitol is usually handled and stored by a health care provider. If you are using Mannitol at home, store Mannitol as directed by your pharmacist or health care provider. Keep Mannitol out of the reach of children and away from pets.
General information:
•If you have any questions about Mannitol, please talk with your doctor, pharmacist, or other health care provider.
•Mannitol is to be used only by the patient for whom it is prescribed. Do not share it with other people.
•If your symptoms do not improve or if they become worse, check with your doctor.
•Check with your pharmacist about how to dispose of unused medicine.
This information is a summary only. It does not contain all information about Mannitol. If you have questions about the medicine you are taking or would like more information, check with your doctor, pharmacist, or other health care provider.
原产地英文商品名:
MANNITOL 25% 50ML/VIAL 25VIALS/BOX
原产地英文药品名:
MANNITOL
中文参考商品译名:
甘露醇25% 50毫升/瓶 25瓶/盒
中文参考药品译名:
甘露醇
产地国家: 美国
所属类别: 泌尿生殖系统及泌乳药物->利尿及脱水药物
处方药:处方药
包装规格: 50毫升/瓶 25瓶/盒
计价单位: 盒
原产地国家批准上市年份:
0000/00/00
英文适应病症1:
Tissue dehydration
英文适应病症2:
Reduce intraocular pressure
英文适应病症3:
Osmotic diuresis
英文适应病症4:
Rinse agent
英文适应病症5:
Preoperative bowel preparation
临床试验期:
完成
中文适应病症参考翻译1:
组织脱水
中文适应病症参考翻译2:
降低眼内压
中文适应病症参考翻译3:
渗透性利尿
中文适应病症参考翻译4:
冲洗剂
中文适应病症参考翻译5:
术前肠道准备