|
美国食品和药物管理局近日批准舒尼替尼(Sunitinib,商品名索坦Sutent)用于治疗不能手术切除肿瘤或肿瘤已扩散至身体的其他部位(转移性)的进展性胰腺神经内分泌癌患者。 这是第二个新获FDA批准用于治疗此种疾病的药物,此前(5月5日)获该机构批准的是依维莫司(everolimus,Afinitor)。舒尼替尼已获美国FDA批准用于治疗晚期肾癌(转移性肾细胞癌)和胃肠道间质瘤(GIST)的患者。胃肠道间质瘤是胃、肠、或食管罕见的癌症。 FDA药物评价和研究中心肿瘤药品办公室主任Richard Pazdur医学博士说,FDA认为重要的是,能给癌症患者提供尽可能多的治疗选择。该机构致力于与公司合作,推进创新疗法进入市场,并鼓励公司继续探索获批产品的其他用途。 胰腺中的神经内分泌肿瘤罕见且生长缓慢。据估计,美国每年新发病例不足1000例。 舒尼替尼的安全性和有效性是立足于一个171例患者的单项研究。纳入研究的患者患转移性(晚期)或局部晚期(不能进行手术切除)的胰腺神经内分泌癌,他们接受舒尼替尼或安慰剂(糖丸)治疗。此项研究旨在评测定患者在疾病扩散或恶化前的生存时间,即无进展生存期。 研究结果表明,舒尼替尼治疗组将疾病扩散或恶化前的生存时间均值延长了10.2个月,而安慰剂组延长了5.4个月。 使用舒尼替尼治疗胰腺神经内分泌癌的患者中,最常见的副作用包括腹泻、恶心、呕吐、乏力、厌食、血压升高、无力、胃/腹痛,头发变色,口腔炎症(口腔炎)和抗感染的白细胞减少(中性粒细胞减少)。 舒尼替尼由位于纽约市的辉瑞制药公司上市销售 Sunitinib Malate CAS: 341031-54-7 Systematic (IUPAC) name
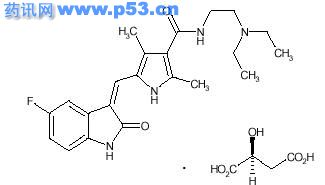
N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide
The molecular formula is C22H27FN4O2•C4H6O5 and the molecular weight is 532.6 Daltons.
The chemical structure of sunitinib malate is:
Sunitinib malate is a yellow to orange powder with a pKa of 8.95. The solubility of sunitinib malate in aqueous media over the range pH 1.2 to pH 6.8 is in excess of 25 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7 is 5.2. Sunitinib (marketed as Sutent, and previously known as SU11248) is an oral, small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) on January 26, 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications. Sunitinib has since become the standard of care for both of these cancers, and is currently being studied for the treatment of many others. |