|
英文药名: Samsca(Tolvaptan Tablets) 中文药名: 托伐普坦片 生产品牌药厂家: Otsuka America Pharma Inc. 药品介绍 美国FDA于2009年5月19日批准了日本大冢制药(Otsuka Pharm)的新分子化合药——托伐普坦片(Tolvaptan Tablets,商品名:Samsca)用于治疗高容或等容性低钠血症伴心力衰竭、肝硬化、抗利尿激素分泌异常综合征。 【英文名】tolvaptan Tablet 【适应症】治疗由充血性心衰、肝硬化以及抗利尿激素分泌不足综合征导致的低钠血症。 【用法用量】15mg/片,一次/日。或遵医嘱。 【药理毒理】一种血管加压素V2受体拮抗药(非肽类AVP2受体拮抗剂),可以升高血浆中钠离子浓度,帮助多余的水分从尿液排出。增强肾脏处理水的能力。多囊肾细胞内环磷酸腺苷(cAMP)积聚,其通过刺激囊液分泌和内衬细胞增生促进囊肿生长。托伐普坦是2型加压素受体拮抗剂,可抑制cAMP生成和聚积。在多种PKD动物模型研究中,托伐普坦显示出良好疗效。 【不良反应】口干、渴感、晕眩、恶心、低血压等。 【规格】15mg;30mg;60mg/片 【生产企业】日本大冢制药公司 Drug: Samsca (tolvaptan) SAMSCA® (tolvaptan)
SAMSCA1 is indicated for the treatment of clinically significant hypervolemic and euvolemic hyponatremia (serum sodium <125 mEq/L or less marked hyponatremia that is symptomatic and has resisted correction with fluid restriction), including patients with heart failure, cirrhosis, and Syndrome of Inappropriate Antidiuretic Hormone (SIADH). Patients requiring intervention to raise serum sodium urgently to prevent or to treat serious neurological symptoms should not be treated with SAMSCA. It has not been established that raising serum sodium with SAMSCA provides a symptomatic benefit to patients. IMPORTANT SAFETY INFORMATION SAMSCA should be initiated and re-initiated in patients only in a hospital where serum sodium can be monitored closely. Too rapid correction of hyponatremia (e.g., >12 mEq/L/24 hours) can cause osmotic demyelination resulting in dysarthria, mutism, dysphagia, lethargy, affective changes, spastic quadriparesis, seizures, coma and death. In susceptible patients, including those with severe malnutrition, alcoholism or advanced liver disease, slower rates of correction may be advisable. SAMSCA is contraindicated in the following conditions: - Urgent need to raise serum sodium acutely · Too Rapid Correction of Serum Sodium Can Cause Serious Neurologic Sequelae – During initiation and after titration monitor patients to assess serum sodium concentrations and neurologic status. Subjects with SIADH or very low baseline serum sodium concentrations may be at greater risk for too-rapid correction of serum sodium. In patients receiving SAMSCA who develop too rapid a rise in serum sodium, discontinue or interrupt treatment with SAMSCA and consider administration of hypotonic fluid. Fluid restriction during the first 24 hours with SAMSCA may increase the likelihood of overly-rapid correction of serum sodium, and should generally be avoided · Gastrointestinal Bleeding in Patients with Cirrhosis – Use in cirrhotic patients only when need to treat outweighs this risk · Dehydration and Hypovolemia – In patients who develop medically significant signs or symptoms of hypovolemia, discontinuation is recommended. Dehydration and hypovolemia can occur, especially in potentially volume-depleted patients receiving diuretics or those who are fluid restricted · Co-administration with Hypertonic Saline – Not recommended · Other Drugs Affecting Exposure to SAMSCA - CYP 3A Inhibitors – Do not use with strong inhibitors of CYP 3A; avoid concomitant use with moderate CYP 3A inhibitors - CYP 3A Inducers – Avoid concomitant use with CYP 3A inducers. If co-administered, the dose of SAMSCA may need to be increased - P-gp Inhibitors – The dose of SAMSCA may have to be reduced if co-administered with P-gp inhibitors · Hyperkalemia or Drugs that Increase Serum Potassium – Monitor serum potassium levels in patients with a serum potassium >5 mEq/L and in patients receiving drugs known to increase serum potassium levels Pregnancy and Nursing Mothers – SAMSCA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from SAMSCA, a decision should be made to discontinue nursing or SAMSCA, taking into consideration the importance of SAMSCA to the mother. Commonly observed adverse reactions – (SAMSCA incidence ≥5% more than placebo, respectively): thirst (16% vs 5%), dry mouth (13% vs 4%), asthenia (9% vs 4%), constipation (7% vs 2%), pollakiuria or polyuria (11% vs 3%) and hyperglycemia (6% vs 1%). *FDA批准托伐普坦片治疗低钠血症 FDA 已经批准托伐普坦片(tolvaptan,Samsca)治疗低钠血症,本品是唯一获准治疗该症的口服型选择性加压素拮抗剂。Samsca在美国地区的销售由Otsuka美国分公司负责。 FDA 新药审评中心心血管与肾病产品的主任Norman Stockbridge 医学博士对外发表声明时称:随着Samsca的获准,医生对治疗低钠血症又多了一种新的选择,本品被用来治疗由充血性心衰、肝硬化以及抗利尿激素分泌不足综合征导致的低钠血症。当血浆中钠离子浓度降低时,为了保持细胞内外的钠离子浓度平衡,细胞外的液体就会进入细胞内,这样细胞就会肿胀。当脑细胞肿胀时,就会导致各种低钠血的症状出现。包括头昏、虚弱、头痛、恶心、意识错乱以及意识减缩和惊厥发生。严重的低钠血症会导致昏迷和死亡, 目前Samsca在重度低血钠患者中还无相应的研究。 Samsca 可以升高血浆中钠离子浓度, 帮助多余的水分从尿液排出。在临床研究中,本品与安慰剂相比,明显升高了患者血浆中的钠离子浓度。本品的黑框警告为患者必须在血钠浓度密切的监控的医院里服用。因为血钠浓度如果升高的过快将导致严重的渗透性脱髓鞘综合征出现。 FDA已经批准托伐普坦片(tolvaptan,Samsca)治疗低钠血症,本品是唯一获准治疗该症的口服型选择性加压素拮抗剂。 托伐普坦片可用来治疗由充血性心衰、肝硬化以及抗利尿激素分泌不足综合征导致的低钠血症。FDA新药审评中心心血管与肾病产品的主任NormanStockbridge医学博士对外发表声明时称:随着托伐普坦片的获准,医生对治疗低钠血症又多了一种新的选择, 当血浆中钠离子浓度降低时,为了保持细胞内外的钠离子浓度平衡,细胞外的液体就会进入细胞内,这样细胞就会肿胀。当脑细胞肿胀时,就会导致各种低钠血的症状出现。包括头昏、虚弱、头痛、恶心、意识错乱以及意识减缩和惊厥发生。严重的低钠血症会导致昏迷和死亡,目前托伐普坦片在重度低血钠患者中还无相应的研究。 托伐普坦片可以升高血浆中钠离子浓度,帮助多余的水分从尿液排出。在临床研究中,本品与安慰剂相比,明显升高了患者血浆中的钠离子浓度。托伐普坦片的黑框警告为患者必须在血钠浓度密切的监控的医院里服用。因为血钠浓度如果升高的过快将导致严重的渗透性脱髓鞘综合征出现。 托伐普坦是由Otsuka公司开发非肽类AVP2受体拮抗剂,仅需一日1次口服。近年来报道的多项针对本品用于CHF治疗的临床随机对照研究均提示,口服托伐普坦能明显减轻患者体重和水肿,且不破坏血电解质平衡,并能有效升高CHF患者并发的低血钠。托伐普坦耐受性好,治疗中不必限制水的摄入。常见不良反应为口干、渴感、晕眩、恶心、低血压等。 サムスカ錠15mg欧文商標名
Samsca tablets
一般名:トルバプタン〔Tolvaptan (JAN)〕
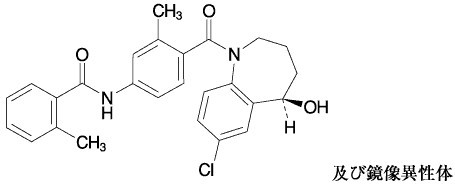
化学名:N-{4-[(5RS)-7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-benzo[b]azepine-1-carbonyl]-3-methylphenyl}-2-methylbenzamide
|
托伐普坦片|Samsca(Tolvaptan Tablets)简介:
英文药名: Samsca(Tolvaptan Tablets)
中文药名: 托伐普坦片
生产品牌药厂家: Otsuka America Pharma Inc.
药品介绍
美国FDA于2009年5月19日批准了日本大冢制药(Otsuka Pharm)的新分子化合药——托 ... 责任编辑:admin |
最新文章更多推荐文章更多热点文章更多
|