|
英文药名:Fostair NEXThaler(Beclometasona/formoterol powder inhaler formulation) 中文药名:丙酸倍氯米松无水/富马酸福莫特罗二水吸入粉 生产厂家:Chiesi Farmaceutici S.p.A.
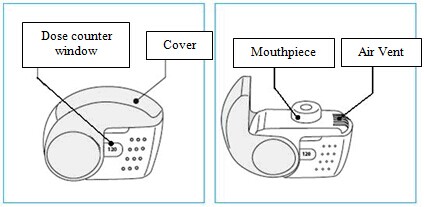
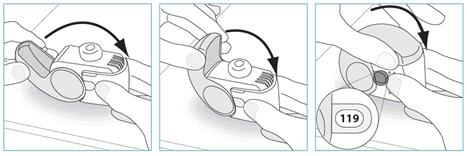
Among the observed adverse reactions those typically associated with beclometasone dipropionate are: nasopharyngitis, oral candidiasis, dysphonia, throat irritation, irritability, cortisol free urine decreased, blood cortisol decreased, blood glucose increased. Additional adverse reactions not observed in the clinical experience with Fostair NEXThaler but typically associated with the inhaled administration of beclometasone dipropionate are other oral fungal infections. Taste disturbances have occasionally been reported during inhaled corticosteroid therapy. See section 4.4 for measures to minimize the occurrence of oral fungal infections, oral candidiasis and dysphonia. Systemic effects of inhaled corticosteroids (e.g. beclometasone dipropionate) may occur particularly when administered at high doses prescribed for prolonged periods, these may include Cushing's Syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, growth retardation in children and adolescents, cataract and glaucoma (see also section 4.4). Additional adverse reactions not observed in the clinical experience with therapeutic doses of Fostair NEXThaler but typically associated with the administration of beta2-agonist such as formoterol are palpitations, atrial fibrillation, ventricular extrasystoles, tachyarrhythmia, potentially serious hypokalaemia and increase/decrease of blood pressure. Insomnia, dizziness, restlessness, and anxiety have occasionally been reported during inhaled formoterol therapy. Formoterol may also induce muscle cramps, myalgia. Hypersensitivity reactions including rashes, urticaria, pruritus and erythema and oedema of the eye, face, lips and throat (angioedema) have been reported. As with other inhalation therapy paradoxical bronchospasm may occur with an immediate increase in wheezing, cough and shortness of breath after dosing (see also section 4.4). Paediatric population There is no information on the safety of Fostair NEXThaler in children up to 11 years of age, and only limited information in adolescents 12 – 17 years of age. In a 12 weeks randomised clinical trial in adults and adolescents, 162 adolescents aged 12 – 17 years with moderate to severe asthma received Fostair NEXThaler or the corresponding pressurised inhalation solution formulation, 1 or 2 inhalations bid; the frequency, type and severity of adverse drug reactions were not different in adolescents compared to adults. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard. 4.9 Overdose The highest recommended dose of Fostair NEXThaler in a single administration is 2 inhalations. Four cumulative inhalations of Fostair NEXThaler (total beclometasone dipropionate 400 micrograms, formoterol 24 micrograms given as a single dose) have been studied in asthmatic patients. The cumulative treatment did not cause abnormal, clinically relevant effect on vital signs and neither serious nor severe adverse reactions were observed (see also section 4.8). For the pressurised inhalation solution formulation, inhaled doses of up to twelve cumulative actuations (total beclometasone dipropionate 1200 micrograms, formoterol 72 micrograms) have been studied in asthmatic patients. The cumulative treatments did not cause abnormal effect on vital signs and neither serious nor severe adverse events were observed. Excessive doses of formoterol may lead to effects that are typical of beta2-adrenergic agonists: nausea, vomiting, headache, tremor, somnolence, palpitations, tachycardia, ventricular arrhythmias, prolongation of QTc interval, metabolic acidosis, hypokalaemia, hyperglycaemia. In case of overdose of formoterol, supportive and symptomatic treatment is indicated. Serious cases should be hospitalised. Use of cardioselective beta-adrenergic blockers may be considered, but only subject to extreme caution since the use of beta-adrenergic blocker medication may provoke bronchospasm. Serum potassium should be monitored. Acute inhalation of beclometasone dipropionate doses in excess of those recommended may lead to temporary suppression of adrenal function. This does not need emergency action as adrenal function recovers in a few days, as verified by plasma cortisol measurements. In these patients treatment should be continued at a dose sufficient to control asthma. Chronic overdose of inhaled beclometasone dipropionate: risk of adrenal suppression (see section 4.4.). Monitoring of adrenal reserve may be necessary. Treatment should be continued at a dose sufficient to control asthma. 5. Pharmacological properties 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Adrenergics, inhalants: formoterol and other drugs for obstructive airway diseases. ATC-code: R03AK08. Mechanisms of action and pharmacodynamic effects Fostair NEXThaler contains beclometasone dipropionate and formoterol in a dry powder formulation resulting in an extrafine aerosol with an average mass median aerodynamic diameter (MMAD) of 1.4-1.5 micrometers and co-deposition of the two components. The aerosol particles of Fostair NEXThaler are on average much smaller than the particles delivered in non-extrafine formulations. A radio-labelled drug deposition study in asthmatic adults has demonstrated that a high proportion of the drug (estimated 42% of the nominal dose) is deposited in the lung, with a homogenous deposition through the airways. These delivery characteristics support the use of a low corticosteroid dose with enhanced local pharmacodynamic effects, which were shown to be equivalent to the corresponding pressurised inhalation solution (see Clinical experience). The two actives of Fostair NEXThaler have different modes of action. In common with other inhaled corticosteroids and beta2-agonist combinations, additive effects are seen in respect of reduction in asthma exacerbations. Beclometasone dipropionate Beclometasone dipropionate given by inhalation at recommended doses has a glucocorticoid antiinflammatory action within the lungs, resulting in reduced symptoms and exacerbations of asthma with less adverse effects than when corticosteroids are administered systemically. Formoterol Formoterol is a selective beta2-adrenergic agonist that produces relaxation of bronchial smooth muscle in patients with reversible airways obstruction. The bronchodilating effect sets in rapidly, within 1-3 minutes after inhalation, and has a duration of 12 hours after dose administration. Clinical experience The efficacy of the two components of Fostair NEXThaler inhalation powder has been assessed in three separate studies in comparison with the 100 micrograms/6 micrograms pressurised inhalation solution formulation in moderate to severe patients with persistent asthma. Overall, the efficacy of the two inhalers is expected to be equivalent in clinical practice at both 1 and 2 inhalations bid. In one study the primary objective was the efficacy evaluation of the inhaled corticosteroid component measured on bronchodilation (pre-dose FEV1). A clinically significant improvement in pre-dose FEV1 was seen in 696 patients with moderate to severe symptomatic asthma at the end of a 3 months treatment period in comparison with baseline values, with 1 inhalation bid and 2 inhalations bid of both formulations. A mean increase of at least 250 mL was observed. There was no clinically relevant difference in pre-dose FEV1 between Fostair NEXThaler inhalation powder and the pressurised inhalation solution at either dosage. A significant dose-response was observed for morning PEF. Statistical significance for the dose-response in pre-dose FEV1 was not reached. Measurements of control of asthma such as morning and evening asthma symptoms scores and percentage of days without symptoms improved significantly from baseline through to the end of the treatment period, particularly for the two high doses of both formulations. In the second study the primary aim was the efficacy evaluation on the long-acting beta2-agonist component of Fostair NEXThaler. In this study bronchodilation at the onset and up to 12 hrs after single doses administration was measured by serial spirometric evaluations of FEV1 (FEV1 AUC over at least 80% of formoterol duration of action). Compared with placebo, Fostair NEXThaler, one inhalation and four inhalations of both actives significantly improved the FEV1 AUC0-12. Both doses of Fostair NEXThaler inhalation powder were non-inferior to the corresponding dose of the pressurised inhalation solution formulation. A statistically significant dose-response was found with both formulations between the low and high dose. In the third study, after a 4-week run-in period with beclometasone dipropionate/formoterol pressurised inhalation solution fixed combination, 1 inhalation bid, 755 controlled asthmatic patients were randomised to 8 weeks of treatment with the same inhaler, with Fostair NEXThaler inhalation powder or with beclometasone dipropionate 100 micrograms per dose inhalation powder, all given at 1 inhalation bid. The primary objective was the change from baseline over the entire treatment period in mean morning expiratory flow (PEF). After 8 weeks of treatment there was no difference in the primary endpoint between the two combination inhalers, both being significantly better than beclometasone dipropionate monotherapy. No differences were found between the two combination inhalers in measures of symptoms such as the asthma control questionnaire score and the number of rescue-free days. An open-label placebo study was conducted to verify that the inspiratory flow which could be generated through the NEXThaler inhaler is not influenced by patient's age, disease and disease severity, and therefore the activation and drug delivery from the device could be achieved in all patients. The primary endpoint was the percentage of patients in each age and disease group able to activate the inhaler. Eighty-nine patients, in the age range 5-84 years, including moderate and severe asthmatics (FEV1 > 60% and ≤ 60% predicted, respectively), and moderate and severe COPD patients (FEV1 ≥ 50% and < 50% predicted, respectively) participated in the trial. All patients, irrespective of age, disease and disease severity, were able to generate sufficient inspiratory flow to activate the NEXThaler inhaler. Paediatric population The European Medicines Agency has deferred the obligation to submit the results of studies in asthma with Fostair NEXThaler in the 5-11 and 12-17 years subsets of the paediatric population. At time of writing, there is no clinical experience on Fostair NEXThaler in children 5 - 11 years of age, and only limited information in adolescents 12 – 17 years of age. In a 3 months randomised clinical trial 162 adolescents aged 12 – 17 years with a diagnosis of moderate to severe asthma received either Fostair NEXThaler or the corresponding pressurised inhalation solution formulation, 1 or 2 inhalations bid. The change in pre-dose FEV1 at the end of treatment was greater in the adolescents than in adults. See also sections 4.2 and 4.8 for information on paediatric use. 5.2 Pharmacokinetic properties Beclometasone dipropionate Beclometasone dipropionate is a pro-drug with weak glucocorticoid receptor binding affinity that is hydrolysed via esterase enzymes to an active metabolite beclometasone-17-monopropionate which has a more potent topical anti-inflammatory activity compared with the pro-drug beclometasone dipropionate. Absorption, distribution and metabolism Inhaled beclometasone dipropionate is rapidly absorbed through the lungs; prior to absorption there is extensive conversion to its active metabolite beclometasone-17-monopropionate via esterase enzymes that are found in most tissues. The systemic availability of the active metabolite arises from lung and from gastrointestinal absorption of the swallowed dose. The bioavailability of swallowed beclometasone dipropionate is negligible however, pre-systemic conversion to beclometasone-17-monopropionate results in part of the dose being absorbed as the active metabolite. There is an approximately linear increase in systemic exposure with increasing inhaled dose. The absolute bioavailability following inhalation from a pressurised metered dose inhaler is approximately 2% and 62% of the nominal dose for unchanged beclometasone dipropionate and beclometasone-17-monopropionate respectively. Following intravenous dosing, the disposition of beclometasone dipropionate and its active metabolite are characterised by high plasma clearance (150 and 120 L/h respectively), with a small volume of distribution at steady state for beclometasone dipropionate (20L) and larger tissue distribution for its active metabolite (424L). Metabolic disposition of beclometasone dipropionate mainly (82%) results in its active metabolite beclometasone-17-monopropionate. Plasma protein binding is moderately high (87%). Excretion Faecal excretion is the major route of beclometasone dipropionate elimination mainly as polar metabolites. The renal excretion of beclometasone dipropionate and its metabolites is negligible. The terminal elimination half-lives are 0.5 h and 2.7 h for beclometasone dipropionate and beclometasone-17-monopropionate respectively. Special populations The pharmacokinetics of beclometasone dipropionate in patients with renal or hepatic impairment has not been studied; however, as beclometasone dipropionate undergoes a very rapid metabolism via esterase enzymes present in intestinal fluid, serum, lungs and liver, to originate the more polar products beclometasone-21-monopropionate, beclometasone-17-monopropionate and beclometasone, hepatic impairment is not expected to modify the pharmacokinetics and safety profile of beclometasone dipropionate. As beclometasone dipropionate or its metabolites were not traced in the urine, an increase in systemic exposure is not envisaged in patients with renal impairment. Formoterol Absorption and distribution Following inhalation, formoterol is absorbed both from the lung and from the gastrointestinal tract. The fraction of an inhaled dose that is swallowed after administration with a metered dose inhaler (MDI) may range between 60% and 90%. At least 65% of the fraction that is swallowed is absorbed from the gastrointestinal tract. Peak plasma concentrations of unchanged drug occur within 0.5 to 1 hours after oral administration. Plasma protein binding of formoterol is 61-64% with 34% bound to albumin. There was no saturation of binding in the concentration range attained with therapeutic doses. The elimination half-life determined after oral administration is 2-3 hours. Absorption of formoterol is linear following inhalation of 12 to 96 μg of formoterol fumarate. Metabolism Formoterol is widely metabolised and the prominent pathway involves direct conjugation at the phenolic hydroxyl group. Glucuronide acid conjugate is inactive. The second major pathway involves O-demethylation followed by conjugation at the phenolic 2'-hydroxyl group. Cytochrome P450 isoenzymes CYP2D6, CYP2C19 and CYP2C9 are involved in the O-demethylation of formoterol. Liver appears to be the primary site of metabolism. Formoterol does not inhibit CYP450 enzymes at therapeutically relevant concentrations. Excretion The cumulative urinary excretion of formoterol after single inhalation from a dry powder inhaler increased linearly in the 12 – 96 μg dose range. On average, 8% and 25% of the dose was excreted as unchanged and total formoterol, respectively. Based on plasma concentrations measured following inhalation of a single 120 μg dose by 12 healthy subjects, the mean terminal elimination half-life was determined to be 10 hours. The (R,R)- and (S,S)-enantiomers represented about 40% and 60% of unchanged drug excreted in the urine, respectively. The relative proportion of the two enantiomers remained constant over the dose range studied and there was no evidence of relative accumulation of one enantiomer over the other after repeated dosing. After oral administration (40 to 80 μg), 6% to 10% of the dose was recovered in urine as unchanged drug in healthy subjects; up to 8% of the dose was recovered as the glucuronide. A total 67% of an oral dose of formoterol is excreted in urine (mainly as metabolites) and the remainder in the faeces. The renal clearance of formoterol is 150 ml/min. Special populations Hepatic/Renal impairment: the pharmacokinetics of formoterol has not been studied in patients with hepatic or renal impairment. Clinical experience The systemic exposure to beclometasone dipropionate and formoterol in the combination has been compared to the single components. There was no evidence of pharmacokinetic or pharmacodynamic (systemic) interactions between beclometasone dipropionate and formoterol. The pharmacokinetics of Fostair NEXThaler inhalation powder has been compared with that of the corresponding pressurised inhalation solution formulation. The analysis of the steroid component focused on beclometasone-17-monopropionate, the main active metabolite of beclometasone dipropionate. Systemic absorption and metabolism of beclometasone dipropionate was rapid and Cmax was reached 5 min postdose for both treatments but was higher (+ 68 %) with Fostair NEXThaler inhalation powder. AUCt was about 3 times higher after inhalation of Fostair NEXThaler compared with the pressurised inhalation solution. Cmax for beclometasone-17-monopropionate, the main active metabolite, representing about 82% of the total blood level, was reached on average after 30 min and 15 min with the NEXThaler and with the pressurised inhalation solution, respectively. Plasma concentration of beclometasone-17-monopropionate was lower (Cmax -49% and AUCt - 29%), after inhalation of the inhalation powder than via the pressurised inhalation solution. After inhalation of Fostair NEXThaler, the peak concentration (Cmax) of formoterol was reached within 5 minutes and was higher (+ 47 %) for the inhalation powder, whereas the overall exposure (AUCt) was comparable in the two treatments. In one study the relative lung delivery was investigated by using a charcoal blockade to exclude drug absorption from the gastrointestinal tract, and adopting an approved spacer, the AeroChamber Plus® for the reference product (the pressurised inhalation solution). In this setting, the NEXThaler and the pressurised inhalation solution were shown to be equivalent for the AUCt of both beclometasone-17-monopropionate and formoterol (the ratio inhalation powder/pressurised inhalation solution and the 90% confidence intervals were within 80-125%); however, Cmax of beclometasone-17-monopropionate was lower (-38%) following inhalation from the NEXThaler. 5.3 Preclinical safety data Non-clinical data of the individual components of Fostair NEXThaler reveal no special hazard for humans based on conventional studies of safety pharmacology and repeated dose toxicity. The toxicity profile of the combination reflected that of single components with no increase in toxicity or unexpected findings. Reproduction studies in rats showed dose-dependent effects. The presence of beclometasone dipropionate at high doses was associated with reduced female fertility, decrease in the number of implantations and embryofetal toxicity. High doses of corticosteroids to pregnant animals are known to cause abnormalities of fetal development including cleft palate and intra-uterine growth retardation, and it is likely that the effects seen with the beclometasone dipropionate/formoterol combination were due to beclometasone dipropionate. These effects were noted only with high systemic exposure to the active metabolite beclometasone-17-monopropionate (more than 200 fold the expected plasma levels in patients). Additionally, increased duration of gestation and parturition, an effect attributable to the known tocolytic effects of beta2-sympathomimetics, was seen in animal studies. These effects were noted when maternal plasma formoterol levels were below the levels expected in patients treated ith Fostair NEXThaler. Genotoxicity studies performed with a beclometasone dipropionate/formoterol combination do not indicate mutagenic potential. No carcinogenicity studies have been performed with the proposed combination. However animal data reported for the individual constituents do not suggest any potential risk of carcinogenicity in man. 6. Pharmaceutical particulars 6.1 List of excipients Lactose monohydrate (which contains small amounts of milk proteins) magnesium stearate. 6.2 Incompatibilities Not applicable. 6.3 Shelf life 3 years. After first opening the pouch, the medicinal product should be used within 6 months. 6.4 Special precautions for storage Store in the original package in order to protect from moisture. Only remove the inhaler from its foil package immediately before first use. Before first opening the pouch: This medicinal product does not require any special temperature storage conditions. After first opening the pouch: Do not store above 25°C. 6.5 Nature and contents of container Each carton contains 1, 2 or 3 NEXThaler inhalers which contain 1.50 g inhalation powder and provide 120 inhalations each. Each inhaler is contained in a heat sealed protective pouch (foil package) made of PET/Al/PE (Polyethylene Terephtalate/Aluminium/ Polyethylene) or PA/Al/PE (Polyamide/Aluminium/Polyethylene). Not all pack sizes may be marketed. Fostair NEXThaler is a multi-dose inhalation device. The device consists of a casework comprising a lower shell with window to display number of doses left and an integral cover. When opened, the cover, which also drives the dose counter mechanism, reveals a mouthpiece through which the drug is inhaled. The lower shell and mouthpiece are made from acrylonitrile butadiene styrene and the cover is made from polypropylene. 6.6 Special precautions for disposal and other handling Any unused product or waste material should be disposed of in accordance with local requirements. Instructions for use of the NEXThaler inhaler are provided below for the benefit of health professionals. INSTRUCTIONS FOR USE OF NEXThaler INHALER A. Contents of the Package This package contains: • 1 instruction leaflet • 1 NEXThaler inhaler inside the sealed protective pouch. If the package contents are not the same as this, return your inhaler to the person who supplied it and get a new one. B. General Warnings & Precautions • Do not remove the inhaler from the pouch if you do not intend to use it immediately. • Only use your inhaler as indicated. • If you are not sure the dose counter has gone down by one after inhalation, wait until your next scheduled dose and take this as normal. Do not take an extra dose. • Keep the cover closed until you need to take a dose from your inhaler. • When you are not using your inhaler keep it in a clean and dry place. • Do not attempt to take your NEXThaler inhaler apart for any reason. • Do not use your NEXThaler inhaler: o after its expiry date o if it is more than 6 months since you opened the pouch o if it is broken o if the dose counter window shows “0” o if you cannot read the dose counter window. In these cases, dispose of your inhaler, or return it to the person who supplied it, and get a new one. Ask your pharmacist how to dispose of inhalers no longer required. C. Key features of your NEXThaler inhaler
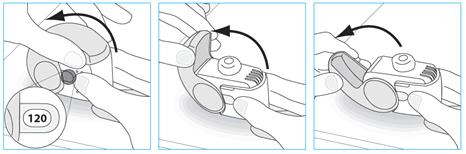
3. Before inhaling breathe out as far as is comfortable.
|
Fostair NEXThaler(丙酸倍氯米松无水/富马酸福莫特罗二水吸入粉)简介:
英文药名:Fostair NEXThaler(Beclometasona/formoterol powder inhaler formulation)
中文药名:丙酸倍氯米松无水/富马酸福莫特罗二水吸入粉
生产厂家:Chiesi Farmaceutici S.p.A.药品介绍治疗适 ... 责任编辑:admin |
最新文章更多推荐文章更多热点文章更多 |