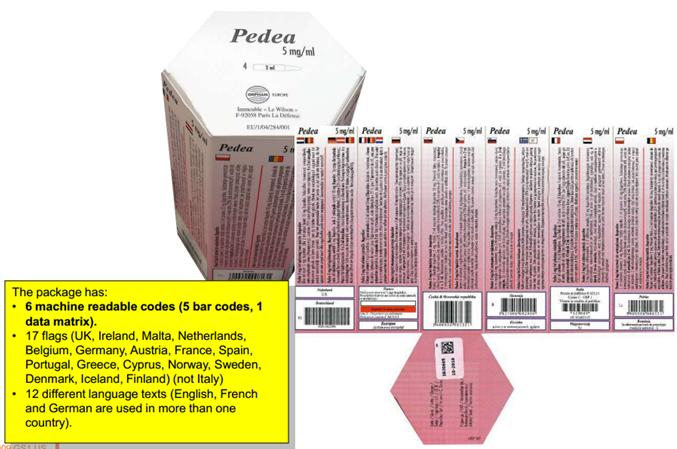
|
部份Pedea中文资料(仅供参考/仅用于医院)
In a clinical trial where Pedea was administered prophylactically during the first 6 hours of life, severe hypoxemia with pulmonary hypertension was reported in 3 newborn infants less than 28 weeks of gestational age. This occurred within one hour of the first infusion and was reversed within 30 minutes after the inhalation of nitric oxide. There have also been post-marketing reports of pulmonary hypertension where Pedea was administered to premature neonates in the therapeutic setting. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via United Kingdom Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard Ireland IMB Pharmacovigilance Earlsfort Terrace Dublin 2 Ireland Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.imb.ie e-mail: imbpharmacovigilance@imb.ie 4.9 Overdose No case of overdose has been reported with intravenous ibuprofen in preterm newborn infants. However, overdose has been described in infants and children administered oral ibuprofen: CNS depression, seizures, gastrointestinal disturbances, bradycardia, hypotension, apnoea, abnormal renal function, haematuria have been observed. Massive overdose (up to more than 1000 mg/kg) has been reported to induce coma, metabolic acidosis, and transient renal failure. All patients recovered with conventional treatment. Only one recorded death has been published: after an overdose of 469 mg/kg, a 16-month old child developed an apnoeic episode with seizures and a fatal aspiration pneumonia. The management of ibuprofen overdose is primarily supportive. 5. Pharmacological properties 5.1 Pharmacodynamic properties Pharmacotherapeutic group: other cardiac preparations, ATC code: C01 EB16 Ibuprofen is a NSAID that possesses anti-inflammatory, analgesic and antipyretic activity. Ibuprofen is a racemic mixture of S(+) and R(-) enantiomers. In vivo and in vitro studies indicate that the S(+) isomer is responsible for the clinical activity. Ibuprofen is a non selective inhibitor of cyclo-oxygenase, leading to reduced synthesis of prostaglandins. Since prostaglandins are involved in the persistence of the ductus arteriosus after birth, this effect is believed to be the main mechanism of action of ibuprofen in this indication. In a dose-response study of Pedea in 40 preterm newborn infants, the ductus arteriosus closure rate associated to the 10-5-5 mg/kg dose regimen was 75% (6/8) in neonates of 27-29 weeks' gestation and 33% (2/6) in neonates of 24-26 weeks' gestation. Prophylactic use of Pedea in the first 3 days of life (starting within 6 hours of birth) in preterm newborn infants less than 28 weeks of gestational age was associated with increased incidence of renal failure and pulmonary adverse events including hypoxia, pulmonary hypertension, pulmonary haemorrhage, as compared to curative use. Conversely, a lower incidence of neonatal grade III-IV intraventricular haemorrhage and of surgical ligation was associated with prophylactic use of Pedea. 5.2 Pharmacokinetic properties Distribution Although a great variability is observed in the premature population, peak plasma concentrations are measured around 35-40 mg/l after the initial loading dose of 10 mg/kg as well as after the last maintenance dose, whatever gestational and postnatal age. Residual concentrations are around 10-15 mg/l 24 hours after the last dose of 5 mg/kg. Plasma concentrations of the S-enantiomer are much higher than those of the R-enantiomer, which reflects a rapid chiral inversion of the R- to the S-form in a proportion similar to adults (about 60%). The apparent volume of distribution is on average 200 ml/kg (62 to 350 according to various studies). The central volume of distribution may depend on the status of the ductus and decrease as the ductus closes. In vitro studies suggest that, similarly to other NSAIDs, ibuprofen is highly bound to plasma albumin, although this seems to be significantly lower (95 %) compared with adult plasma (99 %). Ibuprofen competes with bilirubin for albumin binding in newborn infant serum and, as a consequence, the free fraction of bilirubin may be increased at high ibuprofen concentrations. Elimination Elimination rate is markedly lower than in older children and adults, with an elimination half-life estimated at approximately 30 hours (16–43). The clearance of both enantiomers increases with gestational age, at least in the range of 24 to 28 weeks. PK-PD relationship In preterm newborns ibuprofen significantly reduced plasma concentrations of prostaglandins and their metabolites, particularly PGE2 and 6-keto-PGF-1-alpha. Low levels were sustained up to 72 hours in neonates who received 3 doses of ibuprofen, whereas subsequent re-increases were observed at 72 hours after only 1 dose of ibuprofen. 5.3 Preclinical safety data There are no preclinical data considered relevant to clinical safety beyond data included in other sections of this Summary of Product Characteristics. With the exception of an acute toxicity study, no further studies have been carried out in juvenile animals with Pedea. 6. Pharmaceutical particulars 6.1 List of excipients Trometamol, sodium chloride, sodium hydroxide (for pH adjustment), hydrochloric acid 25% (for pH adjustment), water for injections. 6.2 Incompatibilities This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6. Pedea solution must not be in contact with any acidic solution such as certain antibiotics or diuretics. A rinse of the infusion line must be performed between each product administration (see section 6.6). 6.3 Shelf life 4 years. To avoid any possible microbiological contamination, the product should be used immediately after first opening. 6.4 Special precautions for storage This medicinal product does not require any special storage conditions. 6.5 Nature and contents of container 2 ml solution in a colourless type 1 glass ampoule. Pedea is supplied in packs of 4 x 2 ml ampoules. 6.6 Special precautions for disposal and other handling As for all parenteral products, ampoules of Pedea should be visually inspected for particulate matter and the integrity of the container prior to use. Ampoules are intended for single use only, any unused portions must be discarded. Chlorhexidine must not be used to disinfect the neck of the ampoule as it is not compatible with the Pedea solution. Therefore, for asepsis of the ampoule before use, ethanol 60% or isopropyl alcohol 70% is recommended. When disinfecting the neck of the ampoule with an antiseptic, to avoid any interaction with the Pedea solution, the ampoule must be completely dry before it is opened. The required volume to be given to the infant should be determined according to body weight, and should be injected intravenously as a short infusion over 15 minutes, preferably undiluted. Use only sodium chloride 9 mg/ml (0.9%) solution for injection or glucose 50 mg/ml (5%) solution to adjust injection volume. The total volume of solution injected to preterm infants should take into account the total daily fluid volume administered. A maximal volume of 80 ml/kg/day on the first day of life should usually be respected; this should be progressively increased in the following 1-2 weeks (about 20 ml/kg birthweight/day) up to a maximal volume of 180 ml/kg birthweight/day. Before and after administration of Pedea, to avoid contact with any acidic solution, rinse the infusion line over 15 minutes with 1.5 to 2 ml of either sodium chloride 9 mg/ml (0.9%) or glucose 50 mg/ml (5%), solution for injection. After first opening of an ampoule, any unused portions must be discarded. Any unused product or waste material should be disposed of in accordance with local requirements. 7. Marketing authorisation holder Orphan Europe SARL Immeuble “Le Wilson” 70, avenue du Général de Gaulle F-92800 Puteaux France 8. Marketing authorisation number(s) EU/1/04/284/001 9. Date of first authorisation/renewal of the authorisation Date of first authorisation: 29 July 2004 Date of renewal: 29 July 2009 10. Date of revision of the text 19/12/2013 | ||||||||||||||
布洛芬注射溶液|Pedea(Ibuprofen solution for injection)简介:
部份Pedea中文资料(仅供参考/仅用于医院)中文药名:布洛芬静脉注射剂英文药名:Pedea(Ibuprofen)活性物质布洛芬。药量可作为注射剂注入静脉。•剂量是因人而异的,由孩子的体重来确定。•治 ... 责任编辑:admin
|
最新文章更多推荐文章更多热点文章更多
|