|
盐酸丙哌维林缓释胶囊治疗膀胱过度活动症(OAB)的临床研究,膀胱过度活动症主要病症为:尿频、尿急,伴有或不伴有急迫性尿失禁 MICTONORM® PROPIVERINE HYDROCLORIDE Coated tablet Each sugar-coated contain 15 mg propiverine hydrochloride equivalent to 13.64 mg propiverine.
CLINICAL PHARMACOLOGY Mechanism of action Inhibition of calcium influx and modulation of intracellular calcium in urinary bladder smooth muscle cells causing musculotropic spasmolysis. inhibition of the efferent connection of the nervus pelvicus due to anticholinergic action.
Pharmacodynamic effects In animal models propiverine hydrochloride causes a dose-dependent decreases of the intravesical pressure and an increase in bladder capacity. the effect is based on the sum of the phamacological properties of propiverine and three active urinary metabolites as shown in isolated detrusor strips of human and animal origin.
Pharmacokinetics General characteristics of the active substance propiverine is nearly completely absorbed from the gastrointestinal tract. It undergoes extensive first pass metabolism. Effect of urinary bladder smooth muscle cells are due to the parent compound and three avtive metabolites as well, which are rapidly exreted into the urine.
Absorption after the oral administration of mictonorm® 15 mg Coated Tablets propiverine is rapidly absorbed from the gastrointestinal tract with maximal plasma concentrations reached after 2.3 hours. the mean absolute bioavailability of mictonorm® 15 mg Coated Tablets is 40.5 % (arithmetic mean value for AUC 0-∞ (p.o.)/AUC 0-∞ (i.v.)). Food intake increases the bioavailability of propiverine (mean increase 1.3 fold), but does not significantly affect the maximum plasma concentrations of propiverine or of its main metabolite, propiverine-N-oxide. This difference in bioavailibility is unlikely to be of clinical significance and adjustment of dose in relation to food intake is not required.
Distribution after administration of Mictonorm® 15 mg Coated Tablets t.i.d., steady state is reached after four to five days at higher concentration level than after single dose application (Caverage= 61 ng/ml). The volume of distribution was estimated in 21 healthy volunteers after intravenous administration of propiverine hydrochloride to range from 125 to 4731 (mean 2791) indicating, that a large amount of available propiverine is distributed to peripheral compartements. The binding to plasma proteins is 90-95% for the parent compound and about 60% for the main metabolite.
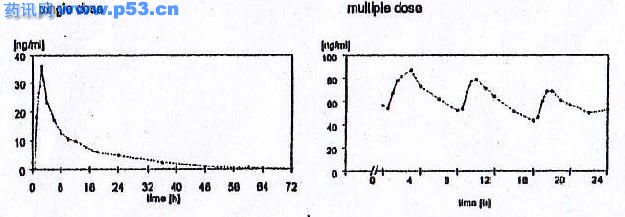
Plasma concentrations of propiverine in 16 healthy volunteera after single and repeated administration of Mictonorm® 15 mg Coated Tablets (l.l.d for 6 days) :
Steady state characteristics of propierine following multiple-dose administration to 16 healthy volunteers of Mictonom® 15 mg Coated Tablets (l.l.d for 6 days) :
Biotransformation Propiverine is extensively metabolised by intestinal and hepatic enzymes. the primary metabolic route involves the oxidation of the Piperidyl-N and is mediated by CYP 3A4 and Flavin-monoxygenases (FMO) 1 and 3 and leads to the formation of the much less active N-oxide, the plasma concentration of which greatly exceeds that of the parent substance. Four metabolites were identified in urine; two of them are pharmacologically active and may contribute to the therapeutic efficacy of Mictonorm® 15 mg Coated Tablets. In vitro there is slight inhibition of CYP 3A4 and CYP 2D6 detectable which occurs at concentrations exceeding therapeutic plasma concentrations 10- to 100-fold (see interaction with medical products and other forms of interactions).
Elimination Following administration of 30 mg oral dose of 14C-propiverine hydrochloride to healthy volunteers, 60% of radio activity was recovered in urine and 21% was recovered in faeces clearance after single dose administration of 30 mg is 371 ml/min (191-870 ml/min). In three studies including a total of 37 healthy volunteers the mean elimination half-life was 14.1, 20.1, and 22.1 hours, respectively.
Linearity/non-linearity pharmacokinetic parameters of propiverine and propiverine-N-oxide following oral administration of 10-30 mg of propiverine hydrochloride are linearly related to dose. There are no changes of pharmacokinetics during steady state compare to single dose administration.
Characteristics in patients Renal impairment: Severe renal impairment does not significantly after the disposition of propiverine and its main metabolite, propiverine-N-oxide, as deduced from the single dose study in 12 patients with creatinine clearance < 30 ml/min. No dose adjustment is to be recommended as long as the total daily dose does not exceed 30 mg (i.e. 45 Mictonorm® 15 mg Coated Tablets given b.i.d.). In case that higher dose (i.e.45 mg) shall be administered a careful titration of dose is recommended considering anticholinergic effects as a marker for tolerability. Hepatic insufficiency: There were similar steady state pharmacokinetics in 12 patients with mild to moderate impairment of liver function due to fatty liver disease as compared to 12 healthy controls. No data are available for severe hepatic impairment. Age: The comparison of trough plasma consentrations during steady state (Mictonorm®15 mg Coated Tablets t.i.d. for 28 days) reveals no difference between older patients (60-85 years; mean 68) and young healthy subjects. The ratio of parent drug to metabolite remains unchanged in older patients indicating the metabolic conversion of propiverine to its main metabolite, propiverine-N-oxide, not to be an age-related or limiting step in the overall excretion. Patients with glaucoma: Intraocular pressure in patients with open angle glaucoma and in patients with treated (controlled) angle closure glaucoma is not increased by Mictonorm® 15 mg Coated Tablets t.i.d., as demonstrated by two placebo-controlled studies.
PRECLINICAL SAFETY DATA In long term dose studies in two mammalian species the main treatment related effect were changes in the liver (including elevation of hepatic enzymes). These were characterised by hepatic hypertrophy and fatty degeneration. The fatty degeneration was reversible upon cessation of treatment. In animal studies, skeletal retardation in the offspring occured when the drug was administered orally at high doses to pregnant females. In lactating mammals propiverine hydrocloride was excreted into the milk. There was no evidence of mutagenicity. Carcinogenicity studies in rodents revealed three types of tumours which were considered to be species specific and therefore not of clinical relevance.
THERAPEUTIC INDICATIONS The treatment of urinary incontinence, as well as urgency and frequency in patients who have either idiophatic detrusor overactivity (overactive bladder) or neurogenic detrusor overactivity (detrusor hyperreflexia) from spinal cord injuries, e.g. transverse lesion paraplegia.
CONTRAINDICATIONS The drug is contraindicated in patients who have demonstrated hypersensitivity to the active substance or to any of the excipients and in patients suffering from one of the following disorders:
SPECIAL WARNINGS AND PRECAUTIONS FOR USE The drug should be used with caution in patients suffering from: autonomic neropathy. Symptoms of the following diseases may be aggravated following administration of the drug:
Propiverine, like other anticholinergics, induces mydriasis. Therefore, the risk to indunce acute angle closure glaucoma in individuals predisposed with narrow angles of the anterior chamber may be increased. Drug of this class have been reported to induce or precipitate acute angle-closure glaucoma. Pollakiuria and nocturia due to renal disease or congestive heart failure as well as organic bladder diseases (e.g. urinary tract infections, malignancy) should be ruled out prior to treatment. Due to a lack of Mictonorm® 15 mg Coated Tablets should not be used in children.
Interaction with other medicinal products and other forms of interactions Increased effect due to concomitant medication with tricylic antidepressants (e.g. Imipiramine), tranquillisers (e.g. Benzodiazepines), anticholinergics, amantadine, neuroleptic (e.g. Phenothiazines) and beta-adrenoreceptor agonists (beta-sympathomimetics). Decreased effects due to concomitant medicaton with cholinergic drugs. Reduced blood pressure in patients treated with isoniazid. The effect of prokinetics such as metoclopramide may be decreased. Pharmacokinetic interactions are possible with other drugs metabolised by cytochrome P450 3A4 (CYP 3A4). However, a very pronounced increase of concertations for such drugs is not expected as the effects of propiverine are small compared to classical enzyme inhibitors (e.g. ketoconazole of grapefruit juice). propiverine may be considered as weak inhibitor of cytochrome P450 3A4. Pharmacokinetics studies with patients concomitantly receiving potent CYP 3A4 inhibitors such as azole antifungals (e.g. ketoconazole, itraconazole) or macrolide antibiotics (e.g. erythromycin, clarithromycin) have not been perfomed.
Use during pregnancy an lactation In animal studies, sceletal retardation in the offspring occurred when the drug was administered orally at high doses to pregnant females.The drug was also secreted in to the milk of lactating mammals. Propiverine hydrochloride should therefore not be administered to pregnant or nursing women.
Effects on ability to drive and use machines Propiverine hydrochloride may produce drowsiness and blurred vision. This may impair the patients abillity to exert activities that require mental alertness such as operating a motor vehicle or other machinery, or to exert hazardous work while taking this drugs. sedative drugs may enhance the drowsiness caused by propiverine hydrochloride.
UNDESIRABLE EFFECTS
All undesirable effects are transcient and recede after a dose reduction or termination of the therapy after maximum 1-4 days.
During long-term therapy hepatic enzymes should be monitored because reversible change of liver enzymes might occure in rare cases. Monitoring of intraocular pressure is recommended in patients at risk of developing glaucoma. Particular attention should be paid to the residual urine volume in cases of urinary tract infection.
OVERDOSE SYMPTOMS, EMERGENCY PROCEDURES, ANTIDOTES Overdose with the muscarinic receptor antagonist propiverine hydrochloride can potentially result in central anticholinergic effect, e.g. restlessness, dizzines, vertigo, disorder in speech and vision and muscular weakness. Moreover severe dryness of mucosa, tachycardia and urinary retention may occur. treatment should be symptomatic and supportive. Management of overdose include initation of vomiting or gastric lavage using an oiled tube (attention: dryness of mucosal), followed by symptomatic and supportive treatment as for atropine overdose (e.g. physostigmine) with a dosage of 1.0 to 2.0 mg in adult by slow intravenous injection (may be repeat as necessary to a total of 5 mg). A 14-years old girl who ingested 450 mg propiverine hydrochloride presented with confabulation. The adolescent fully recovered.
POSOLOGY AND METHOD OF ADMINISTRATION Coated tablets for oral application. The recommended daily doses are as follows: Adult: as a standart coated tablet (=15mg propiverine hydrochloride) twice a day in recommended. This may be increased to three times a day. Some patient may already respond to a dosage of 15 mg a day. For neurogenic detrusor over activity a dose of one coate tablet three times a day is recommended. This may be incresed to four times a day if necessary and tolerated (maximum recommended daily dose). Elderly: general there is no special dosage regimen for the elderly (see pharmacokinetics). There is not clinical relevant effect on food on the phamacokinetics of propiverine (see pharmacokinetics). Accordingly, there is no particular recommendation for the intake of propiverine in relation to food. This medicinal product contains 0.61 mg of glucose. accordingly, a daily dose of 2 coated tablets supplies 1.22 mg of glucose.
HOW SUPPLIED PVC/ alumunium blister in carton with 7 coated tablets per blister; and provided in carton of 4 blister. Registration number.........
STORAGE Do not store above 30˚C. HARUS DENGAN RESEP DOKTER
Manufactured by; APOGEPHA Arzneimittel GMbH Dresden, Germany
Imported by: PT.Phapros, Tbk Semarang, Indonesia 药理药动 本品抑制膀胱平滑肌的钙离子内流电流(ID50=18.3μM),钙拮抗作用可能是异搏停的1/10。本品还有抗胆碱作用,对膀胱的毒蕈碱受体亲和性为阿托品的1.5/1000,竞争性抑制乙酰胆碱的收缩。本品使膀胱伸展的同时并不影响盆腔神经向心性活动。当将盆腔神经的膀胱支切断,刺激断端的中枢端不影响远心性神经的活动,刺激断端的远端,因为膀胱收缩受到了抑制,也不引起膀胱收缩,因此,认为本品的作用并非通过抑制神经反射。 适 应 症 本品适应于神经性尿频,不稳定膀胱,膀胱刺激症状(慢性膀胱炎,前列腺炎)等尿频、尿失禁。 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||