英文药名: Calcijex(Calcitriol Injection)
中文药名: 溉纯(骨化三醇注射液)
药品说明
中文通用名称:骨化三醇
英文通用名称:Calcitriol
中文其他名称:三羟维D3, 钙三醇, 溉纯, 钙化三醇, 罗盖全, 骨化三醇注射液, 骨化三醇软胶囊, 盖三淳, 骨化三醇胶丸, 罗盖金, 海卡洛, 骨化三醇软膏, 德凯, 迪可纯, 秀可丝
英文其他名称: Rocaltrol, Calcijex, Calcjex, Rocaltrol Calcijex, Calcitriol Injection, Calcitriol Soft Capsules, Calcitriol Capsules, Hicalol, Calcitriol Soft Capsuls, Calcitriol Ointment, Decostriol, Silkis
产品所属分类:电解质、酸碱平衡及营养药\钙调节药, 其它专科用药\防治骨质疏松症用药\刺激骨形成类
适应症:
1.用于佝偻病,如维生素D依赖性佝偻病、低血磷性维生素D抵抗型佝偻病等。
2.骨质疏松症(主要用于绝经妇女及老年性骨质疏松症)。
3.用于特发性、假性及术后甲状旁腺功能低下。大剂量静脉给药可用于肾衰竭所致假性甲状旁腺功能减退。
4.用于肾性骨营养不良,如慢性肾衰竭患者(尤其是进行血液透析或腹膜透析者)所致肾性骨营养不良。
5.用于骨软化症。
注意事项:
1.禁忌症
(1)对本药或同类药、维生素D及其类似物过敏者。
(2)有维生素D中毒征象者。
(3)高钙血症及与高血钙相关疾病患者。
2.慎用
尚不明确。
3.药物对儿童的影响
儿童用药的安全性和有效性尚未建立,应避免使用。
4.药物对老人的影响
老年患者使用本药剂量无特殊。
5.药物对妊娠的影响
本药动物实验中,给予家兔最大推荐量的2-6倍,胎儿出现外表和骨骼畸形;而给予同样剂量对大鼠没有致畸作用。孕妇用药的安全性尚未确定,应权衡利弊后用药。美国药品和食品管理局(FDA)对本药的妊娠安全性分级为C级。
6.药物对哺乳的影响
本药吸收后可经乳汁分泌,但尚不明确对哺乳妇女有不良反应,故哺乳时应暂停用药。
7.用药前后及用药时应当检查或监测
用药过程中应监测血钙、磷浓度及血尿素氮、肌酸酐水平,同时应监测尿钙、尿肌酸酐。
不良反应:
1.本药不良反应发生率很低,如小剂量(每日小于0.5μg)单独给药,尚未观察到不良反应。
2.注射给药偶有注射部位疼痛、红肿和过敏反应。
3.长期大剂量用药可引起软弱无力、嗜睡、头痛、恶心、呕吐、肌肉酸痛、骨痛、口腔金属味等。
给药说明:
1.应根据患者血钙水平给予本药每日最佳剂量。患者应摄入足够量(不能过量)的钙,一日平均约为800mg(按从食物和药物摄入计),不应超过1000mg,具体情况应个体化。
2.因血钙增高易诱发心律失常,故使用洋地黄类药物的患者应慎用本药,同时应严密监测血钙浓度。
3.青年患者使用本药只限于特发性骨质疏松症、糖皮质激素过多引起的骨质疏松症。
4.肾功能正常的患者使用本药时,应保持适量的水摄入,不能引起脱水。
5.有观点认为使用本药对驾驶车辆及操作机器的安全性影响很小。
6.本药不能与维生素D(给予药理学剂量)及其衍生物制剂合用,以避免引起高维生素D血症、高钙血症等。
7.出现高钙血症时须立即停药,并给予相关处理,待血钙恢复正常后,按末次剂量减半给药。
8.用药过量可引起高血钙、高尿钙和高血磷。晚期可出现畏光、痛痒、高热、烦渴、多尿、夜尿、畏食、体重减轻、性欲减退、(钙化性)结膜炎、胰腺炎、高血压、心律失常、高胆固醇血症、肝功能异常、血尿素氮升高等,罕见严重精神失常。
9.如出现急性药物过量,可考虑处理为:立即停药,并洗胃或诱导呕吐,避免药物被进一步吸收;口服液体石蜡,以促进药物经肠道的排泄;密切监测血钙浓度,如仍高于正常,可使用磷酸盐和皮质类固醇治疗,同时做适当利尿处理。
用法用量:
成人
·常规剂量
·口服给药
1.一般用量为一日0.3-0.5μg,分2次口服。
2.绝经后骨质疏松:一次0.25μg,一日2次。
3.肾性骨营养不良(包括透析患者):初始阶段,一日0.25μg。血钙正常或略低者,隔日0.25μg。如使用2-4周后病情仍无明显改善,则每隔2-4周,一日0.5μg。多数患者最佳用量为一日0.5-1μg。
4.甲状旁腺功能低下、佝偻病:初始剂量一日0.25μg,晨服。如病情仍无明显改善,则每隔2-4周应增加剂量。对甲状旁腺功能低下者,如出现吸收不佳,应给予较大剂量。
·静脉给药
血液透析患者的肾性骨营养不良:初始剂量一次0.5μg(0.01μg/kg),一周3次。如使用2-4周后病情仍无明显改善,可每隔2-4周,一日增加0.25μg。此类患者补钙应个体化。
儿童
·常规剂量
·口服给药
甲状旁腺功能低下:1-5岁,一日0.25-0.75μg;6岁以上,一日0.5-2μg,用量须个体化。
Brand names: Calcijex®, Rocaltrol®
Package Insert Rx only
Calcitriol injection is synthetically manufactured calcitriol and is available as a sterile, isotonic, clear, colorless to yellow, aqueous solution for intravenous injection. Calcitriol Injection is available as 1 mL of solution packaged in a 2 mL vial. Each 1 mL contains calcitriol, 1 mcg; polysorbate 20, 4 mg; sodium chloride 1.5 mg; sodium ascorbate 10 mg added; dibasic sodium phosphate, anhydrous 7.6 mg; monobasic sodium phosphate, monohydrate 1.8 mg; edetate disodium, dihydrate 1.1 mg added. pH 7.2 (6.5 to 8.0).
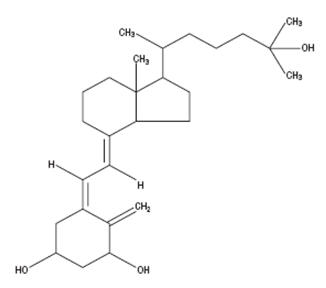
Calcitriol is a crystalline compound, which occurs naturally in humans. It is soluble in organic solvents but relatively insoluble in water. Calcitriol is chemically designated (5Z,7E)-9, 10-secocholesta-5,7,10 (19)-triene-1α,3β,25-triol and has the following structural formula:
Molecular Formula: CHO
Molecular Weight: 416.64
The other names frequently used for calcitriol are 1α,25-dihydroxycholecalciferol, 1α,25-dihydroxyvitamin D, 1,25-DHCC, 1,25(OH)D and 1,25-diOHC.
Calcitriol is the active form of vitamin D (cholecalciferol). The natural or endogenous supply of vitamin D in man mainly depends on ultraviolet light for conversion of 7-dehydrocholesterol to vitamin D in the skin. Vitamin D must be metabolically activated in the liver and the kidney before it is fully active on its target tissues. The initial transformation is catalyzed by a vitamin D-25-hydroxylase enzyme present in the liver, and the product of this reaction is 25-(OH)D (calcifediol). The latter undergoes hydroxylation in the mitochondria of kidney tissue, and this reaction is activated by the renal 25-hydroxyvitamin D-1-α-hydroxylase to produce 1,25-(OH)D (calcitriol), the active form of vitamin D.
The known sites of action of calcitriol are intestine, bone, kidney and parathyroid gland. Calcitriol is the most active known form of vitamin D in stimulating intestinal calcium transport. In acutely uremic rats, calcitriol has been shown to stimulate intestinal calcium absorption. In bone, calcitriol, in conjunction with parathyroid hormone, stimulates resorption of calcium; and in the kidney, calcitriol increases the tubular reabsorption of calcium. In vitro and in vivo studies have shown that calcitriol directly suppresses secretion and synthesis of PTH. A vitamin D-resistant state may exist in uremic patients because of the failure of the kidney to adequately convert precursors to the active compound, calcitriol.
Calcitriol when administered by bolus injection is rapidly available in the blood stream. Vitamin D metabolites are known to be transported in blood, bound to specific plasma proteins. The pharmacologic activity of an administered dose of calcitriol is about 3 to 5 days. Two metabolic pathways for calcitriol have been identified, conversion to 1,24,25-(OH)D and to calcitroic acid.
Calcitriol injection is indicated in the management of hypocalcemia in patients undergoing chronic renal dialysis. It has been shown to significantly reduce elevated parathyroid hormone levels. Reduction of PTH has been shown to result in an improvement in renal osteodystrophy.
Calcitriol injection should not be given to patients with hypercalcemia or evidence of vitamin D toxicity.
Since calcitriol is the most potent metabolite of vitamin D available, vitamin D and its derivatives should be withheld during treatment.
A non-aluminum phosphate-binding compound should be used to control serum phosphorus levels in patients undergoing dialysis.
Overdosage of any form of vitamin D is dangerous (see also OVERDOSAGE ). Progressive hypercalcemia due to overdosage of vitamin D and its metabolites may be so severe as to require emergency attention. Chronic hypercalcemia can lead to generalized vascular calcification, nephrocalcinosis and other soft-tissue calcification. The serum calcium times phosphate (Ca × P) product should not be allowed to exceed 70. Radiographic evaluation of suspect anatomical regions may be useful in the early detection of this condition.
Excessive dosage of calcitriol injection induces hypercalcemia and in some instances hypercalciuria; therefore, early in treatment during dosage adjustment, serum calcium and phosphorus should be determined at least twice weekly. Should hypercalcemia develop, the drug should be discontinued immediately.
Calcitriol injection should be given cautiously to patients on digitalis, because hypercalcemia in such patients may precipitate cardiac arrhythmias.
The patient and his or her parents should be informed about adherence to instructions about diet and calcium supplementation and avoidance of the use of unapproved non-prescription drugs, including magnesium-containing antacids. Patients should also be carefully informed about the symptoms of hypercalcemia (see ADVERSE REACTIONS ).
Serum calcium, phosphorus, magnesium and alkaline phosphatase and 24-hour urinary calcium and phosphorus should be determined periodically. During the initial phase of the medication, serum calcium and phosphorus should be determined more frequently (twice weekly).
Adynamic bone disease may develop if PTH levels are suppressed to abnormal levels. If biopsy is not being done for other (diagnostic) reasons, PTH levels may be used to indicate the rate of bone turnover. If PTH levels fall below recommended target range (1.5 to 3 times the upper limit of normal), in patients treated with calcitriol injection, the calcitriol injection dose should be reduced or therapy discontinued. Discontinuation of calcitriol injection therapy may result in rebound effect, therefore, appropriate titration downward to a maintenance dose is recommended.
Magnesium-containing antacid and calcitriol injection should not be used concomitantly, because such use may lead to the development of hypermagnesemia.
Long-term studies in animals have not been conducted to evaluate the carcinogenic potential of calcitriol injection. Calcitriol was not mutagenic in vitro in the Ames Test nor was oral calcitriol genotoxic in vivo in the Mouse Micronucleus Test. No significant effects on fertility and/or general reproductive performances were observed in a Segment 1 study in rats using oral calcitriol at doses of up to 0.3 mcg/kg.
Calcitriol has been found to be teratogenic in rabbits when given orally at doses of 0.08 and 0.3 mcg/kg. All 15 fetuses in 3 litters at these doses showed external and skeletal abnormalities. However, none of the other 23 litters (156 fetuses) showed external and skeletal abnormalities compared with controls. Teratogenicity studies in rats at doses up to 0.45 mcg/kg orally showed no evidence of teratogenic potential. There are no adequate and well-controlled studies in pregnant women. Calcitriol injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In the rabbit, oral dosages of 0.3 mcg/kg/day administered on days 7 to 18 of gestation resulted in 19% maternal mortality, a decrease in mean fetal body weight and a reduced number of newborns surviving to 24 hours. A study of the effects on orally administered calcitriol on peri- and postnatal development in rats resulted in hypercalcemia in the offspring of dams given calcitriol at doses of 0.08 or 0.3 mcg/kg/day, hypercalcemia and hypophosphatemia in dams given calcitriol at a dose of 0.08 or 0.3 mcg/kg/day and increased serum urea nitrogen in dams given calcitriol at a dose of 0.3 mcg/kg/day. In another study in rats, maternal weight gain was slightly reduced at an oral dose of 0.3 mcg/kg/day administered on days 7 to 15 of gestation.
The offspring of a woman administered oral calcitriol at 17 to 36 mcg/day during pregnancy manifested mild hypercalcemia in the first 2 days of life which returned to normal at day 3.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from calcitriol, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
The safety and effectiveness of calcitriol injection were examined in a 12-week randomized, double-blind, placebo-controlled study of 35 patients, aged 13-18 years, with end-stage renal disease on hemodialysis. Sixty-six percent of the patients were male, 57% were African-American, and nearly all had received some form of vitamin D therapy prior to the study. The initial dose of calcitriol injection was 0.5 mcg, 1 mcg, or 1.5 mcg, 3 times per week, based on baseline iPTH level of less than 500 pg/mL, 500-1000 pg/mL, or greater than 1000 pg/mL, respectively. The dose of calcitriol injection was adjusted in 0.25 mcg increments based on the levels of serum iPTH, calcium and Ca × P. The mean baseline levels of iPTH were 769 pg/mL for the 16 calcitriol injection-treated patients and 897 pg/mL for the 19 placebo-treated subjects. The mean weekly dose of calcitriol injection ranged from 1 mcg to 1.4 mcg. In the primary efficacy analysis, 7 of 16 (44%) subjects in the calcitriol group had 2 consecutive 30% decreases from baseline iPTH compared with 3 of 19 (16%) patients in the placebo group (95% CI for the difference between groups -6%, 62%). One calcitriol injection-treated patient experienced transient hypercalcemia (>11.0 mg/dL), while 6 of 16 (38%) calcitriol injection-treated patients vs. 2 of 19 (11%) placebo-treated patients experienced Ca × P>75.
Clinical studies of calcitriol injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosage range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Adverse effects of calcitriol injection are, in general, similar to those encountered with excessive vitamin D intake. The early and late signs and symptoms of vitamin D intoxication associated with hypercalcemia include:
1. Early
Weakness, headache, somnolence, nausea, vomiting, dry mouth, constipation, muscle pain, bone pain and metallic taste.
2. Late
Polyuria, polydipsia, anorexia, weight loss, nocturia, conjunctivitis (calcific), pancreatitis, photophobia, rhinorrhea, pruritus, hyperthermia, decreased libido, elevated BUN, albuminuria, hypercholesterolemia, elevated SGOT and SGPT, ectopic calcification, hypertension, cardiac arrhythmias and, rarely, overt psychosis.
Occasional mild pain on injection has been observed.
Rare cases of hypersensitivity reactions have been reported, including anaphylaxis.
Administration of calcitriol injection to patients in excess of their requirements can cause hypercalcemia, hypercalciuria and hyperphosphatemia. High intake of calcium and phosphate concomitant with calcitriol injection may lead to similar abnormalities.
General treatment of hypercalcemia (greater than 1 mg/dL above the upper limit of normal range) consists of immediate discontinuation of calcitriol injection therapy, institution of a low calcium diet and withdrawal of calcium supplements. Serum calcium levels should be determined daily until normocalcemia ensues. Hypercalcemia usually resolves in two to seven days. When serum calcium levels have returned to within normal limits, calcitriol injection therapy may be reinstituted at a dose 0.5 mcg less than prior therapy. Serum calcium levels should be obtained at least twice weekly after all dosage changes.
Persistent or markedly elevated serum calcium levels may be corrected by dialysis against a calcium-free dialysate.
The treatment of acute accidental overdosage of calcitriol injection should consist of general supportive measures. Serial serum electrolyte determinations (especially calcium), rate of urinary calcium excretion and assessment of electrocardiographic abnormalities due to hypercalcemia should be obtained. Such monitoring is critical in patients receiving digitalis. Discontinuation of supplemental calcium and low calcium diet are also indicated in accidental overdosage. Due to the relatively short duration of the pharmacological action of calcitriol, further measures are probably unnecessary. Should, however, persistent and markedly elevated serum calcium levels occur, there are a variety of therapeutic alternatives which may be considered, depending on the patients' underlying condition. These include the use of drugs such as phosphates and corticosteroids as well as measures to induce an appropriate forced diuresis. The use of peritoneal dialysis against a calcium-free dialysate has also been reported.
The optimal dose of calcitriol injection must be carefully determined for each patient.
The effectiveness of calcitriol injection therapy is predicated on the assumption that each patient is receiving an adequate and appropriate daily intake of calcium. The RDA for calcium in adults is 800 mg. To ensure that each patient receives an adequate daily intake of calcium, the physician should either prescribe a calcium supplement or instruct the patient in proper dietary measures.
The recommended initial dose of calcitriol injection, depending on the severity of the hypocalcemia and/or secondary hyperparathyroidism, is 1 mcg (0.02 mcg/kg) to 2 mcg administered three times weekly, approximately every other day. Doses as small as 0.5 mcg and as large as 4 mcg three times weekly have been used as an initial dose. If a satisfactory response is not observed, the dose may be increased by 0.5 to 1 mcg at two to four week intervals. During this titration period, serum calcium and phosphorus levels should be obtained at least twice weekly. If hypercalcemia or a serum calcium times phosphate product greater than 70 is noted, the drug should be immediately discontinued until these parameters are appropriate. Then, the calcitriol injection dose should be reinitiated at a lower dose. Doses may need to be reduced as the PTH levels decrease in response to the therapy. Thus, incremental dosing must be individualized and commensurate with PTH, serum calcium and phosphorus levels. The following is a suggested approach in dose titration:
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard unused portion.
| PTH Levels | Calcitriol Injection Dose |
|---|---|
| The same or increasing | increase |
| Decreasing by <30% | increase |
| Decreasing by >30%, <60% | maintain |
| Decreasing by >60% | decrease |
| One and one-half to three times the upper limit of normal | maintain |
Calcitriol Injection is supplied as 1 mL fill in an amber glass vial.
| NDC Number | Contents | Package Size |
|---|---|---|
| 0703-7311-04 | 1 mcg/mL | 2 mL vial packaged 25 per tray |
Protect from light.
Keep vials in tray until time of use.
Store at 20°-25°C (68°-77°F): excursions permitted to 15°- 30°C (59°-86°F). [See USP controlled room temperature.]
Issued: October 2009
Manufactured by:Teva Parenteral Medicines, Inc.Irvine, CA 92618
Y36-X10-434
NDC 0703-7311-04Rx only
Calcitriol Injection
1 mcg/mL
1 mL Single Dose VialsFor IV Injection Only
25 Vials