|
2013年8月10日,赛尔基因(Celgene)的口服抗癌药物pomalidomide已获欧盟委员会(EC)批准,联合地塞米松(dexamethasone)用于既往已接受过至少2次治疗[包括雷利度胺( lenalidomide)和硼替佐米(bortezomib)、且最后一次治疗后经证实病情恶化的复发性和难治性多发性骨髓瘤(rrMM)成人患者的治疗。
To initiate a new cycle of pomalidomide, the neutrophil count must be ≥1 x 109/l and the platelet count must be ≥ 50 x 109/l. In case of neutropaenia, the physician should consider the use of growth factors. For other Grade 3 or 4 adverse reactions judged to be related to pomalidomide, stop treatment and restart treatment at 1 mg less than the previous dose when an adverse reaction has resolved to ≤ Grade 2 at the physician's discretion. If adverse reactions occur after dose reductions to 1 mg, then the medicinal product should be discontinued. • Dexamethasone dose modification instructions
Dose reduction levels (≤ 75 years of age): Starting dose 40 mg; dose level -1 20 mg; dose level-2 10 mg on Days 1, 8, 15 and 22 of each 28-day treatment cycle. Dose reduction levels (> 75 years of age): Starting dose 20 mg; dose level -1 12 mg; dose level-2 8 mg on Days 1, 8, 15 and 22 of each 28-day treatment cycle. If recovery from toxicities is prolonged beyond 14 days, then the dose of dexamethasone will be decreased by one dose level. Special populations Paediatric population There is no relevant use of Imnovid in children aged 0-17 years in the indication of multiple myeloma. Older people No dose adjustment is required for pomalidomide. For patients >75 years of age, the starting dose of dexamethasone is 20 mg once daily on Days 1, 8, 15 and 22 of each 28-day treatment cycle. Renal impairment A study in subjects with renal impairment has not been conducted with pomalidomide. Patients with moderate or severe renal impairment (creatinine clearance <45 mL/min) were excluded from clinical studies. Patients with renal impairment should be carefully monitored for adverse reactions. Hepatic impairment A study in subjects with hepatic impairment has not been conducted with pomalidomide. Patients with serum total bilirubin > 2.0 mg/dL were excluded from clinical studies. Patients with hepatic impairment should be carefully monitored for adverse reactions. Method of administration Oral use. Imnovid should be taken at the same time each day. The capsules should not be opened, broken or chewed (see section 6.6). This medicinal product should be swallowed whole, preferably with water, with or without food. If the patient forgets to take a dose of Imnovid on one day, then the patient should take the normal prescribed dose as scheduled on the next day. Patients should not adjust the dose to make up for a missing dose on previous days. 4.3 Contraindications - Pregnancy. - Women of childbearing potential, unless all the conditions of the pregnancy prevention programme are met (see sections 4.4 and 4.6). - Male patients unable to follow or comply with the required contraceptive measures (see section 4.4). - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1. 4.4 Special warnings and precautions for use Teratogenicity Pomalidomide must not be taken during pregnancy, since a teratogenic effect is expected. Pomalidomide is structurally related to thalidomide. Thalidomide is a known human teratogen that causes severe life-threatening birth defects. Pomalidomide was found to be teratogenic in both rats and rabbits when administered during the period of major organogenesis (see section 5.3). The conditions of the Pregnancy Prevention Programme must be fulfilled for all patients unless there is reliable evidence that the patient does not have childbearing potential. Criteria for women of non-childbearing potential A female patient or a female partner of a male patient is considered of non-childbearing potential if she meets at least one of the following criteria: • Age ≥ 50 years and naturally amenorrhoeic for ≥ 1 year* • Premature ovarian failure confirmed by a specialist gynaecologist • Previous bilateral salpingo-oophorectomy, or hysterectomy • XY genotype, Turner syndrome, uterine agenesis. *Amenorrhoea following cancer therapy or during breast-feeding does not rule out childbearing potential. Counselling For women of childbearing potential, pomalidomide is contraindicated unless all of the following are met: • She understands the expected teratogenic risk to the unborn child • She understands the need for effective contraception, without interruption, 4 weeks before starting treatment, throughout the entire duration of treatment, and 4 weeks after the end of treatment • Even if a woman of childbearing potential has amenorrhoea she must follow all the advice on effective contraception • She should be capable of complying with effective contraceptive measures • She is informed and understands the potential consequences of pregnancy and the need to rapidly consult if there is a risk of pregnancy • She understands the need to commence contraceptive measures as soon as pomalidomide is dispensed following a negative pregnancy test • She understands the need and accepts to undergo pregnancy testing every 4 weeks except in case of confirmed tubal sterilisation • She acknowledges that she understands the hazards and necessary precautions associated with the use of pomalidomide. The prescriber must ensure that for women of childbearing potential: • The patient complies with the conditions of the Pregnancy Prevention Programme, including confirmation that she has an adequate level of understanding • The patient has acknowledged the aforementioned conditions. For male patients taking pomalidomide, pharmacokinetic data has demonstrated that pomalidomide is present in human semen. As a precaution, all male patients taking pomalidomide must meet the following conditions: • He understands the expected teratogenic risk if engaged in sexual activity with a pregnant woman or a woman of childbearing potential • He understands the need for the use of a condom if engaged in sexual activity with a pregnant woman or a woman of childbearing potential not using effective contraception, during treatment and for 7 days after dose interruptions and/or cessation of treatment. Vasectomised males should wear a condom if engaged in sexual activity with a pregnant woman as seminal fluid may still contain pomalidomide in the absence of spermatozoa. • He understands that if his female partner becomes pregnant whilst he is taking pomalidomide or 7 days after he has stopped taking pomalidomide, he should inform his treating physician immediately and that it is recommended to refer the female partner to a physician specialised or experienced in teratology for evaluation and advice. Contraception Women of childbearing potential must use one effective method of contraception for 4 weeks before therapy, during therapy, and until 4 weeks after pomalidomide therapy and even in case of dose interruption unless the patient commits to absolute and continuous abstinence confirmed on a monthly basis. If not established on effective contraception, the patient must be referred to an appropriately trained health care professional for contraceptive advice in order that contraception can be initiated. The following can be considered to be examples of suitable methods of contraception: • Implant • Levonorgestrel-releasing intrauterine system • Medroxyprogesterone acetate depot • Tubal sterilisation • Sexual intercourse with a vasectomised male partner only; vasectomy must be confirmed by two negative semen analyses • Ovulation inhibitory progesterone-only pills (i.e. desogestrel) Because of the increased risk of venous thromboembolism in patients with multiple myeloma taking pomalidomide and dexamethasone, combined oral contraceptive pills are not recommended (see also section 4.5). If a patient is currently using combined oral contraception the patient should switch to one of the effective method listed above. The risk of venous thromboembolism continues for 4−6 weeks after discontinuing combined oral contraception. The efficacy of contraceptive steroids may be reduced during cotreatment with dexamethasone (see section 4.5). Implants and levonorgestrel-releasing intrauterine systems are associated with an increased risk of infection at the time of insertion and irregular vaginal bleeding. Prophylactic antibiotics should be considered particularly in patients with neutropenia. Insertion of copper-releasing intrauterine devices is not recommended due to the potential risks of infection at the time of insertion and menstrual blood loss which may compromise patients with severe neutropenia or severe thrombocytopenia. Pregnancy testing According to local practice, medically supervised pregnancy tests with a minimum sensitivity of 25 mIU/mL must be performed for women of childbearing potential as outlined below. This requirement includes women of childbearing potential who practice absolute and continuous abstinence. Ideally, pregnancy testing, issuing a prescription and dispensing should occur on the same day. Dispensing of pomalidomide to women of childbearing potential should occur within 7 days of the prescription. Prior to starting treatment A medically supervised pregnancy test should be performed during the consultation, when pomalidomide is prescribed, or in the 3 days prior to the visit to the prescriber once the patient had been using effective contraception for at least 4 weeks. The test should ensure the patient is not pregnant when she starts treatment with pomalidomide. Follow-up and end of treatment A medically supervised pregnancy test should be repeated every 4 weeks, including 4 weeks after the end of treatment, except in the case of confirmed tubal sterilisation. These pregnancy tests should be performed on the day of the prescribing visit or in the 3 days prior to the visit to the prescriber. Men Pomalidomide is present in human semen during treatment. As a precaution, and taking into account special populations with potentially prolonged elimination time such as renal impairment, all male patients taking pomalidomide, including those who have had a vasectomy, should use condoms throughout treatment duration, during dose interruption and for 7 days after cessation of treatment if their partner is pregnant or of childbearing potential and has no contraception. Male patients should not donate semen or sperm during treatment (including during dose interruptions) and for 7 days following discontinuation of pomalidomide. Additional precautions Patients should be instructed never to give this medicinal product to another person and to return any unused capsules to their pharmacist at the end of treatment. Patients should not donate blood, semen or sperm during treatment (including during dose interruptions) and for 7 days following discontinuation of pomalidomide. Educational materials, prescribing and dispensing restrictions In order to assist patients in avoiding foetal exposure to pomalidomide, the Marketing Authorisation Holder will provide educational material to health care professionals to reinforce the warnings about the expected teratogenicity of pomalidomide, to provide advice on contraception before therapy is started, and to provide guidance on the need for pregnancy testing. The prescriber must inform the patient about the expected teratogenic risk and the strict pregnancy prevention measures as specified in the Pregnancy Prevention Programme and provide patients with appropriate patient educational brochure, patient card and/or equivalent tool in accordance with the national implemented patient card system. A national controlled distribution system has been implemented in collaboration with each National Competent Authority. The controlled distribution system includes the use of a patient card and/or equivalent tool for prescribing and /or dispensing controls, and the collection of detailed data relating to the indication in order to monitor the off-label use within the national territory. Ideally, pregnancy testing, issuing a prescription and dispensing should occur on the same day. Dispensing of pomalidomide to women of childbearing potential should occur within 7 days of the prescription and following a medically supervised negative pregnancy test result. Prescriptions for women of childbearing potential can be for a maximum duration of 4 weeks, and prescriptions for all other patients can be for a maximum duration of 12 weeks. Haematological events Neutropenia was the most frequently reported Grade 3 or 4 haematological adverse reaction in patients with relapsed/refractory multiple myeloma, followed by anaemia and thrombocytopenia. Patients should be monitored for haematological adverse reactions, especially neutropenia. Patients should be advised to report febrile episodes promptly. Physicians should observe patients for signs of bleeding including epistaxes, especially with use of concomitant medicinal products known to increase the risk of bleeding. Complete blood counts should be monitored at baseline, weekly for the first 8 weeks and monthly thereafter. A dose modification may be required (see section 4.2). Patients may require use of blood product support and /or growth factors. Thromboembolic events Patients receiving pomalidomide in combination with dexamethasone have developed venous thromboembolic events (predominantly deep vein thrombosis and pulmonary embolism) and arterial thrombotic events. Patients with known risk factors for thromboembolism – including prior thrombosis – should be closely monitored. Action should be taken to try to minimise all modifiable risk factors (e.g. smoking, hypertension, and hyperlipidaemia). Patients and physicians are advised to be observant for the signs and symptoms of thromboembolism. Patients should be instructed to seek medical care if they develop symptoms such as shortness of breath, chest pain, arm or leg swelling. Anti-coagulation therapy (unless contraindicated) is recommended, (such as acetylsalicylic acid, warfarin, heparin or clopidogrel), especially in patients with additional thrombotic risk factors. A decision to take prophylactic measures should be made after a careful assessment of the individual patient's underlying risk factors. In clinical studies, patients received prophylactic acetylsalicylic acid or alternative anti-thrombotic therapy. The use of erythropoietic agents carries a risk of thrombotic events including thromboembolism. Therefore, erythropoietic agents, as well as other agents that may increase the risk of thromboembolic events, should be used with caution. Peripheral neuropathy Patients with ongoing ≥Grade 2 peripheral neuropathy were excluded from clinical studies with pomalidomide. Appropriate caution should be exercised when considering the treatment of such patients with pomalidomide. Significant cardiac dysfunction Patients with significant cardiac dysfunction (congestive heart failure [NY Heart Association Class III or IV]; myocardial infarction within 12 months of starting study; unstable or poorly controlled angina pectoris) were excluded from clinical studies with pomalidomide. Cardiac failure events, including congestive cardiac failure and pulmonary oedema (see section 4.8), have been reported, mainly in patients with pre-existing cardiac disease or cardiac risk factors. Appropriate caution should be exercised when considering the treatment of such patients with pomalidomide, including periodic monitoring for signs or symptoms of cardiac failure. Tumour lysis syndrome Tumour lysis syndrome may occur.The patients at greatest risk of tumour lysis syndrome are those with high tumour burden prior to treatment. These patients should be monitored closely and appropriate precautions taken. Second Primary Malignancies Second primary malignancies have been reported in patients receiving pomalidomide. Physicians should carefully evaluate patients before and during treatment using standard cancer screening for occurrence of second primary malignancies and institute treatment as indicated. Allergic reaction Angioedema and severe dermatologic reactions have been reported (see section 4.8). Patients with a prior history of serious allergic reactions associated with thalidomide or lenalidomide were excluded from clinical studies. Such patients may be at higher risk of hypersensitivity reactions and should not receive pomalidomide. Pomalidomide interruption or discontinuation should be considered for Grade 2-3 skin rash. Pomalidomide must be discontinued permanently for angioedema, Grade 4 rash, exfoliative or bullous rash. Dizziness and confusion Dizziness and confusional state have been reported with pomalidomide. Patients must avoid situations where dizziness or confusion may be a problem and not to take other medicinal products that may cause dizziness or confusion without first seeking medical advice. Interstitial lung disease (ILD) ILD and related events, including cases of pneumonitis, have been observed with pomalidomide. Careful assessment of patients with an acute onset or unexplained worsening of pulmonary symptoms should be performed to exclude ILD. Pomalidomide should be interrupted pending investigation of these symptoms and if ILD is confirmed, appropriate treatment should be initiated. Pomalidomide should only be resumed after a thorough evaluation of the benefits and the risks. Hepatic Disorders Markedly elevated levels of alanine aminotransferase and bilirubin have been observed in patients treated with pomalidomide (see section 4.8). There have also been cases of hepatitis that resulted in discontinuation of pomalidomide. Regular monitoring of liver function is recommended for the first 6 months of treatment with pomalidomide and as clinically indicated thereafter. 4.5 Interaction with other medicinal products and other forms of interaction Effect of Imnovid on other medicinal products Pomalidomide is not anticipated to cause clinically relevant pharmacokinetic drug-drug interactions due to P450 isoenzyme inhibition or induction or transporter inhibition when co-administered with substrates of these enzymes or transporters. The potential for such drug-drug interactions, including the potential impact of pomalidomide on the pharmacokinetics of combined oral contraceptives, has not been evaluated clinically (see section 4.4 Teratogenicity). Effect of other medicinal products on Imnovid Pomalidomide is partly metabolised by CYP1A2 and CYP3A4/5. It is also a substrate for P-glycoprotein. Co-administration of pomalidomide with the strong CYP3A4/5 and P-gp inhibitor ketoconazole, or the strong CYP3A4/5 inducer carbamazepine, had no clinically relevant effect on exposure to pomalidomide. Co-administration of the strong CYP1A2 inhibitor fluvoxamine with pomalidomide in the presence of ketoconazole, increased exposure to pomalidomide by 104% with a 90 % confidence interval [88% to 122%] compared to pomalidomide plus ketoconazole. If strong inhibitors of CYP1A2 (e.g. ciprofloxacin, enoxacin and fluvoxamine) are co-administered with pomalidomide, patients should be closely monitored for the occurrence of adverse reactions. Dexamethasone Co-administration of multiple doses of up to 4 mg pomalidomide with 20 mg to 40 mg dexamethasone (a weak to moderate inducer of several CYP enzymes including CYP3A) to patients with multiple myeloma had no effect on the pharmacokinetics of pomalidomide compared with pomalidomide administered alone. The effect of dexamethasone on warfarin is unknown. Close monitoring of warfarin concentration is advised during treatment. 4.6 Fertility, pregnancy and lactation Women of childbearing potential / Contraception in males and females Women of childbearing potential should use effective method of contraception. If pregnancy occurs in a woman treated with pomalidomide, treatment must be stopped and the patient should be referred to a physician specialised or experienced in teratology for evaluation and advice. If pregnancy occurs in a partner of a male patient taking pomalidomide, it is recommended to refer the female partner to a physician specialised or experienced in teratology for evaluation and advice. Pomalidomide is present in human semen. As a precaution, all male patients taking pomalidomide should use condoms throughout treatment duration, during dose interruption and for 7 days after cessation of treatment if their partner is pregnant or of childbearing potential and has no contraception (see sections 4.3 and 4.4). Pregnancy A teratogenic effect of pomalidomide in humans is expected. Pomalidomide is contraindicated during pregnancy and in women of childbearing potential, except when all the conditions for pregnancy prevention have been met, see section 4.3 and section 4.4 Breast-feeding It is not known if pomalidomide is excreted in human milk. Pomalidomide was detected in milk of lactating rats following administration to the mother. Because of the potential for adverse reactions in nursing infants from pomalidomide, a decision should be made whether to discontinue nursing or to discontinue the medicinal product, taking into account the importance of the medicinal product to the mother. Fertility Pomalidomide was found to impact negatively on fertility and be teratogenic in animals. Pomalidomide crossed the placenta and was detected in foetal blood following administration to pregnant rabbits. See section 5.3. 4.7 Effects on ability to drive and use machines Imnovid has minor or moderate influence on the ability to drive and use machines. Fatigue, depressed level of consciousness, confusion, and dizziness have been reported with the use of pomalidomide. If affected, patients should be instructed not to drive cars, use machines or perform hazardous tasks while being treated with pomalidomide. 4.8 Undesirable effects Summary of the safety profile The most commonly reported adverse reactions in clinical studies have been blood and lymphatic system disorders including anaemia (45.7%), neutropenia (45.3%) and thrombocytopenia (27%); in general disorders and administration site conditions including fatigue (28.3%), pyrexia (21%) and oedema peripheral (13%); and in infections and infestations including pneumonia (10.7%). Peripheral neuropathy adverse reactions were reported in 12.3% of patients and venous embolic or thrombotic (VTE) adverse reactions were reported in 3.3% of patients. The most commonly reported Grade 3 or 4 adverse reactions were in the blood and lymphatic system disorders including neutropenia (41.7%), anaemia (27%) and thrombocytopenia (20.7%); in infections and infestations including pneumonia (9%); and in general disorders and administration site conditions including fatigue (4.7%), pyrexia (3%) and oedema peripheral (1.3%). The most commonly reported serious adverse reaction was pneumonia (9.3%). Other serious adverse reactions reported included febrile neutropenia (4.0%), neutropenia (2.0%), thrombocytopenia (1.7%) and VTE adverse reactions (1.7 %). Adverse reactions tended to occur more frequently within the first 2 cycles of treatment with pomalidomide. Tabulated list of adverse reactions In randomised study CC-4047-MM-003, 302 patients with relapsed and refractory multiple myeloma were exposed to 4 mg pomalidomide administered once daily for 21 days of each 28 day cycle in combination with a weekly low dose of dexamethasone. The adverse reactions observed in patients treated with pomalidomide plus dexamethasone are listed below by system organ class (SOC) and frequency for all adverse reactions and for Grade 3 or 4 adverse reactions. The frequencies of adverse reactions are those reported in the pomalidomide plus dexamethasone arm of study CC-4047-MM-003 (n = 302) and from post marketing data. Within each SOC and frequency grouping, adverse reactions are presented in order of decreasing seriousness. Frequencies are defined in accordance with current guidance, as: very common (≥1/10), common (≥1/100 to <1/10); and uncommon (≥1/1,000 to <1/100).
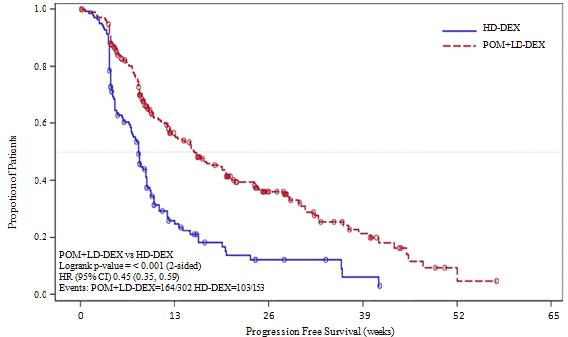
Description of selected adverse reactions Teratogenicity Pomalidomide is structurally related to thalidomide. Thalidomide is a known human teratogenic active substance that causes severe life-threatening birth defects. Pomalidomide was found to be teratogenic in both rats and rabbits when administered during the period of major organogenesis (see sections 4.6 and 5.3). If pomalidomide is taken during pregnancy, a teratogenic effect of pomalidomide in humans is expected (see section 4.4). Neutropenia and thrombocytopenia Neutropenia occurred in 45.3% of patients who received pomalidomide plus low dose dexamethasone (Pom + LD-Dex), and in 19.5% of patients who received high dose dexamethasone (HD-Dex). Neutropenia was Grade 3 or 4 in 41.7% of patients who received Pom + LD-Dex, compared with 14.8% who received HD-Dex. In Pom + LD-Dex treated patients neutropenia was infrequently serious (2.0% of patients), did not lead to treatment discontinuation, and was associated with treatment interruption in 21.0% of patients, and with dose reduction in 7.7% of patients. Febrile neutropenia (FN) was experienced in 6.7% of patients who received Pom + LD-Dex, and in no patients who received HD-Dex. All were reported to be Grade 3 or 4. FN was reported to be serious in 4.0% of patients. FN was associated with dose interruption in 3.7% of patients, and with dose reduction in 1.3% of patients, and with no treatment discontinuations. Thrombocytopenia occurred in 27.0% of patients who received Pom + LD-Dex, and 26.8% of patients who received HD-Dex. Thrombocytopenia was Grade 3 or 4 in 20.7% of patients who received Pom + LD-Dex and in 24.2% who received HD-Dex. In Pom + LD-Dex treated patients, thrombocytopenia was serious in 1.7% of patients, led to dose reduction in 6.3% of patients, to dose interruption in 8% of patients and to treatment discontinuation in 0.7% of patients. (see sections 4.2 and 4.4) Infection Infection was the most common non haematological toxicity; it occurred in 55.0% of patients who received Pom + LD-Dex, and 48.3% of patients who received HD-Dex. Approximately half of those infections were Grade 3 or 4; 24.0% in Pom + LD-Dex-treated patients and 22.8% in patients who received HD-Dex. In Pom + LD-Dex treated patients pneumonia and upper respiratory tract infections were the most commonly reported infections (in 10.7% and 9.3% of patients, respectively);with 24.3% of reported infections being serious and fatal infections (Grade 5) occurring in 2.7% of treated patients. In Pom + LD-Dex treated patients infections led to dose discontinuation in 2.0% of patients, to treatment interruption in 14.3% of patients, and to a dose reduction in 1.3% of patients. Thromboembolic events Venous embolic or thrombotic events (VTE) occurred in 3.3% of patients who received Pom + LD-Dex, and 2.0% of patients who received HD-Dex. Grade 3 or 4 reactions occurred in 1.3 % of patients who received Pom + LD-Dex, and no patients who received HD-Dex. In Pom + LD-Dex treated patients, VTE was reported as serious in 1.7% of patients, no fatal reactions were reported in clinical studies, and VTE was not associated with dose discontinuation. Prophylaxis with acetylsalicylic acid (and other anticoagulants in high risk subjects) was mandatory for all patients in clinical studies. Anticoagulation therapy (unless contraindicated) is recommended (see section 4.4). Peripheral neuropathy Patients with ongoing peripheral neuropathy ≥Grade 2 were excluded from clinical studies. Peripheral neuropathy, mostly Grade 1 or 2 occurred in 12.3% patients who received Pom + LD-Dex, and 10.7% of patients who received HD-Dex. Grade 3 or 4 reactions occurred in 1.0 % of patients who received Pom + LD-Dex and in 1.3% of patients who received HD-Dex. In patients treated with Pom + LD-Dex, no peripheral neuropathy reactions were reported to have been serious in clinical trials and peripheral neuropathy led to dose discontinuation in 0.3% of patients (see section 4.4). Median time to onset of neuropathy was 2.1 weeks, varying from 0.1 to 48.3 weeks. Median time to onset was earlier in patients who received HD-Dex compared with Pom + LD-Dex (1.3 weeks versus 2.1 weeks). Median time to resolution was 22.4 weeks in patients who received Pom + LD-Dex and 13.6 weeks in patients who received HD-Dex. The lower limit of the 95% CI was 5.3 week in the Pom +LD-Dex-treated patients and 2.0 weeks in patients who received HD-Dex. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard (Freephone 0808 100 3352). 4.9 Overdose Imnovid doses as high as 50 mg as a single dose in healthy volunteers, and 10 mg as once-daily multiple doses in patients suffering from multiple myeloma have been studied without reported serious adverse events related to overdose. No specific information is available on the treatment of overdose with pomalidomide, and it is unknown whether pomalidomide or its metabolites are dialysable. In the event of overdose, supportive care is advised. 5. Pharmacological properties 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Immunomodulating agent, ATC code: L04AX06 Mechanism of action Pomalidomide has direct anti-myeloma tumoricidal activity, immunomodulatory activities and inhibits stromal cell support for multiple myeloma tumour cell growth. Specifically, pomalidomide inhibits proliferation and induces apoptosis of haematopoietic tumour cells. Additionally, pomalidomide inhibits the proliferation of lenalidomide-resistant multiple myeloma cell lines and synergises with dexamethasone in both lenalidomide-sensitive and lenalidomide-resistant cell lines to induce tumour cell apoptosis. Pomalidomide enhances T cell- and natural killer (NK) cell-mediated immunity and inhibits production of pro-inflammatory cytokines (e.g., TNF-α and IL-6) by monocytes. Pomalidomide also inhibits angiogenesis by blocking the migration and adhesion of endothelial cells. Clinical efficacy and safety The efficacy and safety of pomalidomide in combination with dexamethasone were evaluated in a Phase III multi-centre, randomised, open-label study (CC-4047-MM-003), where pomalidomide plus low-dose dexamethasone therapy (Pom+LD-Dex) was compared to high-dose dexamethasone alone (HD-Dex) in previously treated adult patients with relapsed and refractory multiple myeloma, who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy. A total of 455 patients were enrolled in the study: 302 in the Pom+LD-Dex arm and 153 in the HD-Dex arm. The majority of patients were male (59%) and white (79%); the median age for the overall population was 64 years (min, max: 35, 87 years). Patients in the Pom+LD-Dex arm were administered 4 mg pomalidomide orally on Days 1 to 21 of each 28-day cycle. LD-Dex (40 mg) was administered once per day on Days 1, 8, 15 and 22 of a 28-day cycle. For the HD-Dex arm, dexamethasone (40 mg) was administered once per day on Days 1 through 4, 9 through 12, and 17 through 20 of a 28-day cycle. Patients > 75 years of age started treatment with 20 mg dexamethasone. Treatment continued until patients had disease progression. The primary efficacy endpoint was progression free survival (PFS) by International Myeloma Working Group (IMWG criteria). For the ITT population, median PFS time by Independent Review Adjudication Committee (IRAC) review based on IMWG criteria was 15.7 weeks (95% CI: 13.0, 20.1) in the Pom + LD-Dex arm; the estimated 26-week event-free survival rate was 35.99% (±3.46%). In the HD-Dex arm, median PFS time was 8.0 weeks (95% CI: 7.0, 9.0); the estimated 26-week event-free survival rate was 12.15% (±3.63%). Progression-free survival was evaluated in several relevant subgroups: gender, race, ECOG performance status, stratification factors (age, disease population, prior anti-myeloma therapies [2, > 2]), selected parameters of prognostic significance (baseline beta-2 microglobulin level, baseline albumin levels, baseline renal impairment, and cytogenetic risk), and exposure and refractoriness to prior anti-myeloma therapies. Regardless of the subgroup evaluated, PFS was generally consistent with that observed in the ITT population for both treatment groups. Progression Free Survival is summarised in Table 1 for the ITT population. Kaplan-Meier curve for PFS for the ITT population is provided in Figure 1. Table 1: Progression Free Survival Time by IRAC Review Based on IMWG Criteria (Stratified Log Rank Test) (ITT Population)
a The median is based on Kaplan-Meier estimate. b 95% confidence interval about the median progression free survival time. c Based on Cox proportional hazards model comparing the hazard functions associated with treatment groups, stratified by age (≤75 vs >75),diseases population (refractory to both Lenalidomide and Bortezomib vs not refractory to both drugs), and prior number of anti myeloma therapy (=2 vs >2). d The p-value is based on a stratified log-rank test with the same stratification factors as the above Cox model. Data cutoff: 07 Sep 2012 Figure 1: Progression Free Survival Based on IRAC Review of Response by IMWG Criteria (Stratified Log Rank Test) (ITT Population
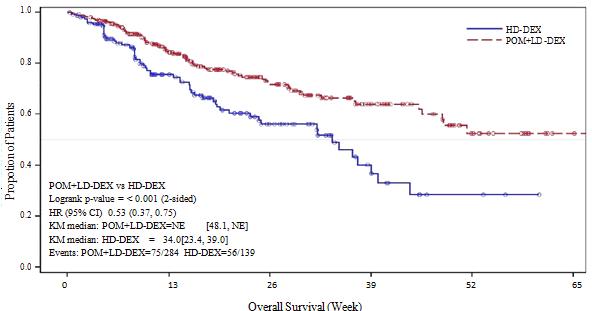
a The median is based on Kaplan-Meier estimate. b 95% confidence interval about the median overall survival time. c Based on Cox proportional hazards model comparing the hazard functions associated with treatment groups. d The p-value is based on an unstratified log-rank test. Data cutoff: 07 Sep 2012 Figure 2: Kaplan-Meier Curve of Overall Survival (ITT Population)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||