|
新型抗真菌药物CRESEMBA(ISAVUCONAZONIUM SULFATE)被美国FDA批准上市,为患者提供一个安全有效的治疗方案,用于治疗侵袭性曲霉菌病和侵袭性毛霉菌病成人患者
b 186 mg of isavuconazonium sulfate is equivalent to 100 mg of isavuconazole c Start maintenance doses 12 to 24 hours after the last loading dose Switching between the intravenous and oral formulations of CRESEMBA is acceptable as bioequivalence has been demonstrated. Loading dose is not required when switching between formulations. With oral administration, swallow capsules whole. Do not chew, crush, dissolve, or open the capsules. CRESEMBA capsules can be taken with or without food. 2.3 Reconstitution Instructions for the Injection Formulation Aseptic technique must be strictly observed in all handling since no preservative or bacteriostatic agent is present in CRESEMBA or in the materials specified for reconstitution. CRESEMBA is water soluble, preservative-free, sterile, and nonpyrogenic. • Reconstitute one vial of CRESEMBA by adding 5 mL water for injection, USP to the vial. • Gently shake to dissolve the powder completely. • Visually inspect the reconstituted solution for particulate matter and discoloration. Reconstituted CRESEMBA should be clear and free of visible particulate. • The reconstituted solution may be stored below 25°C for maximum 1 hour prior to preparation of the patient infusion solution. 2.4 Dilution and Preparation Instructions for the Injection Formulation • Remove 5 mL of the reconstituted solution from the vial and add it to an infusion bag containing 250 mL (approximately 1.5 mg isavuconazonium sulfate per mL) of compatible diluent. The diluted solution may show visible translucent to white particulates of isavuconazole (which will be removed by in-line filtration). • Use gentle mixing or roll bag to minimize the formation of particulates. Avoid unnecessary vibration or vigorous shaking of the solution. • Apply in-line filter with a microporous membrane pore size of 0.2 to 1.2 micron and in-line filter reminder sticker to the infusion bag. • Do not use a pneumatic transport system. • The intravenous administration should be completed within 6 hours of dilution at room temperature. If this is not possible, immediately refrigerate (2° to 8°C / 36° to 46°F) the infusion solution after dilution and complete the infusion within 24 hours. Do not freeze the infusion solution. 2.5 Compatibility for the Injection Formulation CRESEMBA for injection should only be administered with the following diluents: • 0.9% sodium chloride injection, USP • 5% dextrose injection, USP 3 DOSAGE FORMS AND STRENGTHS Each CRESEMBA capsule contains 186 mg isavuconazonium sulfate (equivalent to 100 mg of isavuconazole). Capsules are opaque and elongated, and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a white cap imprinted with “766” in black ink. Each single-dose vial of CRESEMBA for injection contains 372 mg isavuconazonium sulfate (equivalent to 200 mg of isavuconazole). CRESEMBA for injection is supplied in a single-dose vial as a sterile lyophilized white to yellow powder. 4 CONTRAINDICATIONS • CRESEMBA is contraindicated in persons with known hypersensitivity to isavuconazole. • Coadministration of strong CYP3A4 inhibitors, such as ketoconazole or high-dose ritonavir (400 mg every 12 hours), with CRESEMBA is contraindicated because strong CYP3A4 inhibitors can significantly increase the plasma concentration of isavuconazole [see Drug Interactions (7) and Clinical Pharmacology (12.3)]. • Coadministration of strong CYP3A4 inducers, such as rifampin, carbamazepine, St. John’s wort, or long acting barbiturates with CRESEMBA is contraindicated because strong CYP3A4 inducers can significantly decrease the plasma concentration of isavuconazole [see Drug Interactions (7) and Clinical Pharmacology (12.3)]. • CRESEMBA shortened the QTc interval in a concentration-related manner. CRESEMBA is contraindicated in patients with familial short QT syndrome [see Clinical Pharmacology (12.2)]. 5 WARNINGS AND PRECAUTIONS 5.1 Hepatic Adverse Drug Reactions Hepatic adverse drug reactions (e.g., elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin) have been reported in clinical trials. The elevations in liver-related laboratory tests were generally reversible and did not require discontinuation of CRESEMBA. Cases of more severe hepatic adverse drug reactions including hepatitis, cholestasis or hepatic failure including death have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with azole antifungal agents, including CRESEMBA. Evaluate liver-related laboratory tests at the start and during the course of CRESEMBA therapy. Monitor patients who develop abnormal liver-related laboratory tests during CRESEMBA therapy for the development of more severe hepatic injury. Discontinue CRESEMBA if clinical signs and symptoms consistent with liver disease develop that may be attributable to CRESEMBA [see Adverse Reactions (6.1)]. 5.2 Infusion-Related Reactions Infusion-related reactions including hypotension, dyspnea, chills, dizziness, paresthesia, and hypoesthesia were reported during intravenous administration of CRESEMBA. Discontinue the infusion if these reactions occur [see Adverse Reactions (6.1)]. 5.3 Hypersensitivity Reactions Serious hypersensitivity and severe skin reactions, such as anaphylaxis or Stevens Johnson syndrome, have been reported during treatment with other azole antifungal agents. Discontinue CRESEMBA if a patient develops a severe cutaneous adverse reaction. There is no information regarding cross-sensitivity between CRESEMBA and other azole antifungal agents. Caution should be used when prescribing CRESEMBA to patients with hypersensitivity to other azoles. 5.4 Embryo-Fetal Toxicity CRESEMBA may cause fetal harm when administered to a pregnant woman. CRESEMBA should be used during pregnancy only if the potential benefit to the patient outweighs the risk to the fetus. Women who become pregnant while receiving CRESEMBA are encouraged to contact their physician [see Use in Specific Populations (8.1)]. Perinatal mortality was significantly increased in the offspring of pregnant rats dosed orally with isavuconazonium sulfate at 90 mg/kg/day (less than half the maintenance human dose based on AUC comparisons) during pregnancy through the weaning period. Isavuconazonium chloride administration was associated with dose-related increases in the incidences of rudimentary cervical ribs in rats and rabbits at 30 and 45 mg/kg, respectively, doses equivalent to about one fifth and one tenth of the clinical exposures based on AUC comparisons. In rats, dose-related increases in the incidences of zygomatic arch fusion and supernumerary ribs/rudimentary supernumerary ribs were also noted at 30 mg/kg and above, equivalent to one fifth the clinical dose based on AUC comparisons [see Nonclinical Toxicology (13.1)]. 5.5 Drug Interactions Coadministration of CRESEMBA with strong CYP3A4 inhibitors such as ketoconazole or high-dose ritonavir and strong CYP3A4 inducers such as rifampin, carbamazepine, St. John’s wort, or long acting barbiturates is contraindicated [see Contraindications (4) and Drug Interactions (7)]. 5.6 Drug Particulates Following dilution, CRESEMBA intravenous formulation may form precipitate from the insoluble isavuconazole. Administer CRESEMBA through an in-line filter [see Dosage and Administration (2.4)]. 6 ADVERSE REACTIONS The following are discussed in more detail in other sections of the labeling: • Hepatic Adverse Drug Reactions [see Warnings and Precautions (5.1)] • Infusion-Related Reactions [see Warnings and Precautions (5.2)] • Hypersensitivity Reactions [see Warnings and Precautions (5.3)] • Embryo-Fetal Toxicity [see Warnings and Precautions (5.4)] Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of CRESEMBA cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice. 6.1 Clinical Trial Experience A total of 403 patients were exposed to CRESEMBA in two clinical trials. The most frequently reported adverse reactions among CRESEMBA-treated patients were nausea (26%), vomiting (25%), diarrhea (22%), headache (17%), elevated liver chemistry tests (16%), hypokalemia (14%), constipation (13%), dyspnea (12%), cough (12%), peripheral edema (11%), and back pain (10%). Serious adverse reactions occurred in 223/403 (55%) of patients and 56/403 (14%) of patients permanently discontinued treatment with CRESEMBA due to an adverse reaction in the two trials. The adverse reactions which most often led to permanent discontinuation of CRESEMBA therapy during the clinical trials were: confusional state (0.7%), acute renal failure (0.7%), increased blood bilirubin (0.5%), convulsion (0.5%), dyspnea (0.5%), epilepsy (0.5%), respiratory failure (0.5%), and vomiting (0.5%). Patients in the clinical trials were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, graft-versus-host disease, and hematopoietic stem cell transplant. The patient population was 61% male, had a mean age of 51 years (range 17-92, including 85 patients aged greater than 65 years), and was 79% white and 3% black. One hundred forty-four (144) patients had a duration of CRESEMBA therapy of greater than 12 weeks, with 52 patients receiving CRESEMBA for over six months. In Trial 1, a randomized, double-blind, active-controlled clinical trial for treatment of invasive aspergillosis, treatment-emergent adverse reactions occurred in 247/257 (96%), and 255/259 (99%) patients in the CRESEMBA and voriconazole treatment groups, respectively. Treatment-emergent adverse reactions resulting in permanent discontinuation were reported in 37 (14%) CRESEMBA-treated patients and 59 (23%) voriconazole-treated patients. Table 2 includes selected treatment-emergent adverse reactions which were reported at an incidence of ≥ 5% during CRESEMBA therapy in Trial 1. In Trial 2, an open-label, non-comparative trial of CRESEMBA in patients with invasive aspergillosis and renal impairment or invasive mucormycosis, treatment-emergent adverse reactions occurred in 139/146 (95%) of patients in the CRESEMBA treatment group. Adverse reactions resulting in permanent discontinuation were reported in 19 (13%) CRESEMBA-treated patients. The frequencies and types of adverse reactions observed in CRESEMBA-treated patients were similar between Trial 1 and Trial 2. Table 2. Selected Treatment-Emergent Adverse Reactions with Rates of 5% or Greater in CRESEMBA-treated Patients in Trial 1
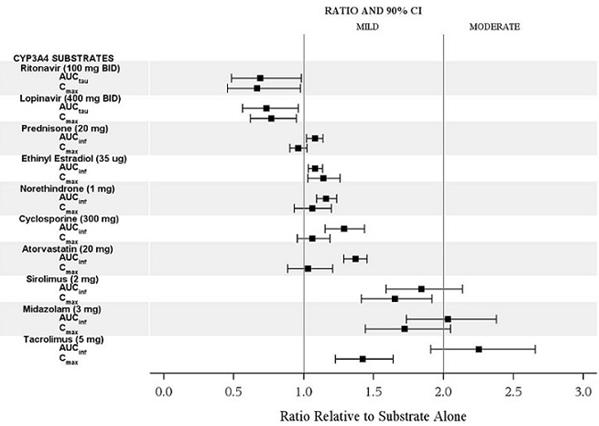
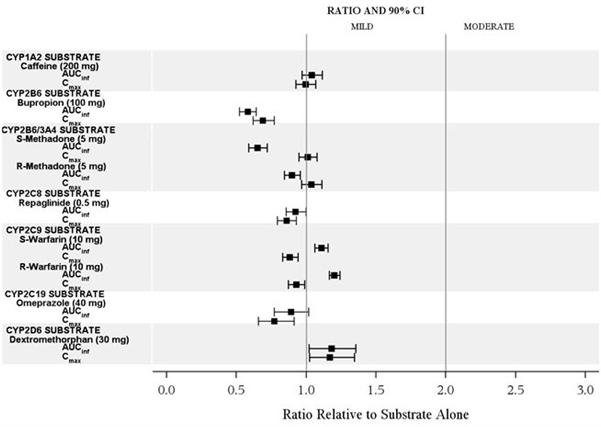
b Delirium includes adverse reactions of agitation, confusional state, delirium, disorientation, and mental status changes. The following adverse reactions occurred in less than 5% of all CRESEMBA-treated patients in Trial 1 or 2. The list does not include reactions presented in Table 2. This listing includes adverse reactions where a causal relationship to isavuconazole cannot be ruled out or those which may help the physician in managing the risks to the patients. Blood and lymphatic system disorders: agranulocytosis, leukopenia, pancytopenia Cardiac disorders: atrial fibrillation, atrial flutter, bradycardia, reduced QT interval on electrocardiogram, palpitations, supraventricular extrasystoles, supraventricular tachycardia, ventricular extrasystoles, cardiac arrest Ear and labyrinth disorders: tinnitus, vertigo Eye disorders: optic neuropathy Gastrointestinal disorders: abdominal distension, gastritis, gingivitis, stomatitis General disorders and administration site conditions: catheter thrombosis, malaise, chills Hepatobiliary disorders: cholecystitis, cholelithiasis, hepatitis, hepatomegaly, hepatic failure Immune system disorders: hypersensitivity Injury, poisoning and procedural complications: fall Metabolism and nutrition disorders: hypoalbuminemia, hypoglycemia, hyponatremia Musculoskeletal and connective tissue disorders: myositis, bone pain, neck pain Nervous system disorders: convulsion, dysgeusia, encephalopathy, hypoesthesia, migraine, peripheral neuropathy, paraesthesia, somnolence, stupor, syncope, tremor Psychiatric disorders: confusion, hallucination, depression Renal and urinary disorders: hematuria, proteinuria Respiratory, thoracic and mediastinal disorders: bronchospasm, tachypnea Skin and subcutaneous tissue disorders: alopecia, dermatitis, exfoliative dermatitis, erythema, petechiae, urticaria Vascular disorders: thrombophlebitis Laboratory effects In Trial 1, elevated liver transaminases (alanine aminotransferase or aspartate aminotransferase) greater than three times the upper limit of normal were reported at the end of study treatment in 4.4% of patients who received CRESEMBA. Elevations of liver transaminases greater than ten times the upper limit of normal developed in 1.2% of patients who received CRESEMBA. 7 DRUG INTERACTIONS Isavuconazole is a sensitive substrate of CYP3A4. CYP3A4 inhibitors or inducers may alter the plasma concentrations of isavuconazole. Isavuconazole is a moderate inhibitor of CYP3A4, and a mild inhibitor of P-glycoprotein (P-gp), and organic cation transporter 2 (OCT2). Drug interaction studies were conducted to investigate the effect of co-administered drugs on pharmacokinetics of isavuconazole and the effect of isavuconazole on the pharmacokinetics of co-administered drugs [see Clinical Pharmacology (12.3)]. Table 3. Drug(s) Affecting Pharmacokinetics of CRESEMBA
Table 4. The Effect of CRESEMBA on the Pharmacokinetics of Other Drugs
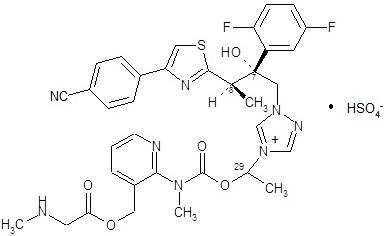
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Pregnancy Category C There are no adequate and well-controlled clinical studies of CRESEMBA in pregnant women. CRESEMBA should be used during pregnancy only if the potential benefit to the patient outweighs the risk to the fetus. Women who become pregnant during CRESEMBA treatment are encouraged to contact their physician. Risk Summary Based on animal data, CRESEMBA is predicted to have the potential for increasing the risk of adverse developmental outcomes above background risk. Animal Data Perinatal mortality was significantly increased in the offspring of pregnant rats dosed orally with isavuconazonium sulfate at 90 mg/kg/day (less than half the maintenance human dose based on AUC comparisons) during pregnancy through the weaning period. Isavuconazonium chloride administration was associated with dose-related increases in the incidences of rudimentary cervical ribs in rats and rabbits at 30 and 45 mg/kg, respectively, doses equivalent to about one fifth and one tenth of the clinical exposures based on AUC comparisons. In rats, dose-related increases in the incidences of zygomatic arch fusion and supernumerary ribs/rudimentary supernumerary ribs were also noted at 30 mg/kg and above, equivalent to one fifth the clinical dose based on AUC comparisons [see Nonclinical Toxicology (13.1)]. Skeletal abnormalities have also been observed in embryo-fetal development studies of other azole antifungal agents. 8.3 Nursing Mothers Isavuconazole is excreted in the milk of lactating rats following intravenous administration. Mothers should not breast feed while taking CRESEMBA. 8.4 Pediatric Use The safety and efficacy of CRESEMBA in pediatric patients less than 18 years of age have not been established. 8.5 Geriatric Use Of the 547 patients who received CRESEMBA in the Phase 2 and 3 trials, 86 (16%) of patients were greater than 65 years of age and 20 (4%) were greater than 75 years of age. The pharmacokinetics of isavuconazole are comparable in young and elderly subjects (65 years of age and older) [see Clinical Pharmacology (12.3)]. No dose adjustment of CRESEMBA is needed in elderly patients. 8.6 Renal Impairment Of the 403 patients who received CRESEMBA in the Phase 3 trials, 79 (20%) of patients had an estimated glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild, moderate, or severe renal impairment, including those patients with End Stage Renal Disease (ESRD) [see Clinical Pharmacology (12.3)]. 8.7 Hepatic Impairment No dose adjustment is necessary in patients with mild or moderate hepatic impairment (Child-Pugh Class A and B) [see Clinical Pharmacology (12.3)]. CRESEMBA has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) and should be used in these patients only when the benefits outweigh the risks. Clinical monitoring for CRESEMBA-related adverse reactions is recommended when treating patients with severe hepatic impairment [see Warnings and Precautions (5.1)]. 10 OVERDOSAGE During clinical studies, total daily CRESEMBA doses higher than the recommended dose regimen were associated with an increased rate of adverse reactions. At supratherapeutic doses (three times the recommended maintenance dose) evaluated in a thorough QT study, there were proportionally more treatment-emergent adverse reactions than in the therapeutic dose group (maintenance dose) for the following: headache, dizziness, paresthesia, somnolence, disturbance in attention, dysgeusia, dry mouth, diarrhea, oral hypoesthesia, vomiting, hot flush, anxiety, restlessness, palpitations, tachycardia, photophobia and arthralgia. Treatment-emergent adverse reactions leading to discontinuation of study drug occurred in 7 of 39 (17.9%) subjects in the supratherapeutic dose group. Isavuconazole is not removed by hemodialysis. There is no specific antidote for isavuconazole. Treatment should be supportive with appropriate monitoring. 11 DESCRIPTION CRESEMBA contains isavuconazonium sulfate, which is the prodrug of isavuconazole, an azole antifungal drug. Isavuconazonium sulfate drug substance is an amorphous, white to yellowish-white powder. The chemical name of isavuconazonium sulfate is glycine, N-methyl-, [2-[[[1-[1-[(2R,3R)-3-[4-(4-cyanophenyl)-2-thiazolyl]-2-(2,5-difluorophenyl)-2-hydroxybutyl]-4H-1,2,4-triazolium-4-yl]ethoxy]carbonyl]methylamino]-3-pyridinyl]methyl ester, sulfate (1:1). The empirical formula is C35H35F2N8O5S·HSO4, the molecular weight is 814.84 and the structural formula is:
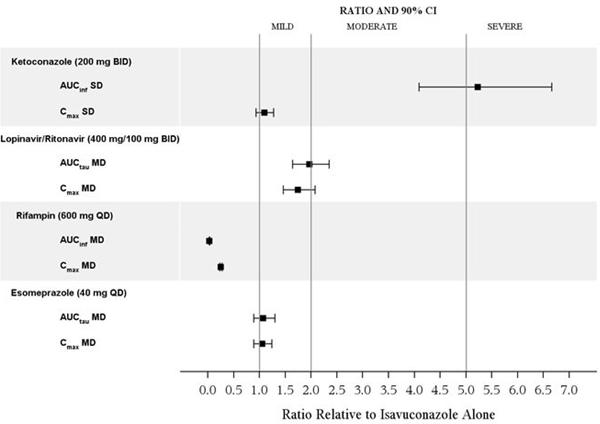
Absorption After oral administration of CRESEMBA in healthy volunteers, the active moiety, isavuconazole, generally reaches maximum plasma concentrations (Cmax) 2 hours to 3 hours after single and multiple dosing. The absolute bioavailability of isavuconazole following oral administration of CRESEMBA is 98%. No significant concentrations of the prodrug or inactive cleavage product were seen in plasma after oral administration. Following intravenous administration of CRESEMBA, maximal plasma concentrations of the prodrug and inactive cleavage product were detectable during infusion and declined rapidly following the end of administration. The prodrug was below the level of detection by 1.25 hours after the start of a 1 hour infusion. The total exposure of the prodrug based on AUC was less than 1% that of isavuconazole. The inactive cleavage product was quantifiable in some subjects up to 8 hours after the start of infusion. The total exposure of inactive cleavage product based on AUC was approximately 1.3% that of isavuconazole. Effect of Food Coadministration of CRESEMBA equivalent to isavuconazole 400 mg oral dose with a high-fat meal reduced isavuconazole Cmax by 9% and increased AUC by 9%. CRESEMBA can be taken with or without food. Distribution Isavuconazole is extensively distributed with a mean steady state volume of distribution (Vss) of approximately 450 L. Isavuconazole is highly protein bound (greater than 99%), predominantly to albumin. Metabolism In in vitro studies isavuconazonium sulfate is rapidly hydrolyzed in blood to isavuconazole by esterases, predominately by butylcholinesterase. Isavuconazole is a substrate of cytochrome P450 enzymes 3A4 and 3A5. Following single doses of [cyano 14C] isavuconazonium and [pyridinylmethyl 14C] isavuconazonium in humans, in addition to the active moiety (isavuconazole) and the inactive cleavage product, a number of minor metabolites were identified. Except for the active moiety isavuconazole, no individual metabolite was observed with an AUC greater than 10% of drug related material. In vivo studies indicate that CYP3A4, CYP3A5 and subsequently uridine diphosphate-glucuronosyltransferases (UGT) are involved in the metabolism of isavuconazole. Excretion Following oral administration of radio-labeled isavuconazonium sulfate to healthy volunteers, a mean of 46.1% of the total radioactive dose was recovered in the feces and 45.5% was recovered in the urine. Renal excretion of isavuconazole itself was less than 1% of the dose administered. The inactive cleavage product is primarily eliminated by metabolism and subsequent renal excretion of the metabolites. Renal elimination of intact cleavage product was less than 1% of the total dose administered. Following intravenous administration of radio-labeled cleavage product, 95% of the total radioactive dose was excreted in the urine. Special populations Geriatric Patients The AUC of isavuconazole following a single oral dose of CRESEMBA equivalent to 200 mg isavuconazole in elderly subjects (65 years and older) was similar to that in younger volunteers (18 years to 45 years). The AUC was similar between younger female and male subjects and between elderly and younger males. Elderly female AUC estimates were 38% and 47% greater than AUC estimates obtained in elderly males and younger females, respectively. The pharmacokinetic difference in elderly females receiving CRESEMBA are not considered to be clinically significant. Therefore, no dose adjustment is required based on age and gender. Pediatric Patients The pharmacokinetics of CRESEMBA in pediatric patients have not been evaluated. Race A 2-compartment population pharmacokinetic model was developed to assess the pharmacokinetics of isavuconazole between healthy Western and Chinese subjects. Chinese subjects were found to have on average a 40% lower clearance compared to Western subjects (1.6 L/hr for Chinese subjects as compared to 2.6 L/hr for Western subjects) and therefore approximately 50% higher AUC than Western subjects. Body mass index (BMI) did not play a role in the observed differences. No dose adjustment is recommended for Chinese patients. Gender AUC estimates were similar between young female and male subjects (18 years to 45 years). There was a difference in AUC for elderly females, see Geriatric section above. No dose adjustment is required based on gender. Renal Impairment Total isavuconazole AUC and Cmax were not affected to a clinically meaningful extent in subjects with mild, moderate and severe renal impairment relative to healthy controls. No dose adjustment is necessary in patients with renal impairment. Isavuconazole is not readily dialyzable. A dose adjustment is not warranted in patients with ESRD. Hepatic Impairment After a single dose of CRESEMBA equivalent to 100 mg of isavuconazole was administered to 32 patients with mild (Child-Pugh Class A) hepatic impairment and 32 patients with moderate (Child-Pugh Class B) hepatic impairment (16 intravenous and 16 oral patients per Child-Pugh Class), the least squares mean systemic exposure (AUC) increased 64% and 84% in the Child-Pugh Class A group and the Child-Pugh Class B group, respectively, relative to 32 age and weight-matched healthy subjects with normal hepatic function. Mean Cmax was 2% lower in the Child-Pugh Class A group and 30% lower in the Child-Pugh Class B group. The population pharmacokinetic evaluation of isavuconazole in healthy subjects and patients with mild and moderate hepatic impairment demonstrated that the mild and moderate hepatic impairment population had 40% and 48% lower isavuconazole clearance (CL) values, respectively, compared to the healthy population. It is recommended that the standard CRESEMBA loading dose and maintenance dose regimen be utilized in patients with mild to moderate hepatic disease. CRESEMBA has not been studied in patients with severe hepatic impairment (Child-Pugh Class C). Drug Interaction Studies Isavuconazole is a substrate of CYP3A4 and CYP3A5. In vitro, isavuconazole is an inhibitor of CYP3A4, CYP2C8, CYP2C9, CYP2C19, and CYP2D6. Isavuconazole is also an inhibitor of P-gp-, BCRP- and OCT2-mediated drug transporters. In vitro, isavuconazole is also an inducer of CYP3A4, CYP2B6, CYP2C8, and CYP2C9. The effect of coadministration of drugs on the pharmacokinetics of isavuconazole and the effect of isavuconazole on the pharmacokinetics of co-administered drugs were studied after single and multiple doses of isavuconazole in healthy subjects. The effects of ketoconazole, rifampin, lopinavir/ritonavir, and esomeprazole on isavuconazole are shown in Figure 1. Ketoconazole: As a strong CYP3A4 inhibitor, ketoconazole increased the isavuconazole Cmax by 9% and isavuconazole AUC by 422% after multiple dose administration of ketoconazole (200 mg twice daily) for 24 days and a single dose of CRESEMBA equivalent to 200 mg of isavuconazole. Isavuconazole is a sensitive CYP3A4 substrate and use with strong CYP3A4 inhibitors are contraindicated per Section 4 and Figure 1. Lopinavir/Ritonavir: Lopinavir/ritonavir (400 mg/100 mg twice daily) increased the Cmax and AUC of isavuconazole (clinical dose) 74% and 96%, respectively, with concurrent decreases in the mean AUCs of lopinavir and ritonavir by 27% and 31%, respectively. Rifampin: Rifampin (600 mg) decreased the mean Cmax and AUC of isavuconazole by 75% and 97%, respectively, when coadministered with multiple doses of CRESEMBA and thus, coadministration of CRESEMBA with strong CYP3A4 inducers is contraindicated.
b Neutropenia defined as less than 500 cells/mm3. Patients randomized to receive CRESEMBA treatment were administered a loading dose intravenously of 372 mg of isavuconazonium sulfate (equivalent to 200 mg of isavuconazole) every 8 hours for the first 48 hours. Beginning on day 3, patients received intravenous or oral therapy of 372 mg of isavuconazonium sulfate (equivalent to 200 mg of isavuconazole) once daily. Patients randomized to receive voriconazole treatment were administered voriconazole intravenously with a loading dose of 6 mg/kg every 12 hours for the first 24 hours followed by 4 mg/kg intravenously every 12 hours for the following 24 hours. Therapy could then be switched to an oral formulation of voriconazole at a dose of 200 mg every 12 hours. In this trial, the protocol-defined maximum treatment duration was 84 days. Mean treatment duration was 47 days for both treatment groups, of which 8 to 9 days was by an intravenous route of administration. All-cause mortality through Day 42 in the overall population (ITT) was 18.6% in the CRESEMBA treatment group and 20.2% in the voriconazole treatment group for an adjusted treatment difference of -1.0% with 95% confidence interval of -8.0% to 5.9%. Similar results were seen in patients with proven or probable invasive aspergillosis confirmed by serology, culture or histology (see Table 7). Table 7. All-Cause Mortality Through Day 42
Overall success at End-of-Treatment (EOT) was assessed by a blinded, independent Data Review Committee (DRC) using pre-specified clinical, mycological, and radiological criteria. In the subgroup of patients with proven or probable invasive aspergillosis confirmed by serology, culture or histology, overall success at EOT was seen in 35% of CRESEMBA-treated patients compared to 38.9% of voriconazole-treated patients (see Table 8). Table 8. Overall Response Success at End-of-Treatment
14.2 Treatment of Invasive Mucormycosis Trial 2, an open-label non-comparative trial, evaluated the safety and efficacy of a subset of patients with invasive mucormycosis. Thirty-seven (37) patients had proven or probable mucormycosis according to criteria based on those established by the European Organisation for Research and Treatment of Cancer/Mycoses Study Group1. Rhizopus oryzae and Mucormycetes were the most common pathogens identified. There were few patients with other Mucorales: Lichtheimia corymbifera, Mucor amphibiorum, Mucor circinelloides, Rhizomucor pusillus, Rhizopus azygosporus, and Rhizopus microsporus. The patients were white (68%), male (81%), and had a mean age of 49 years (range 22-79). Fifty-nine percent (59%) of patients had pulmonary disease involvement, half of whom also had other organ involvement. The most common non-pulmonary disease locations were sinus (43%), eye (19%), CNS (16%) and bone (14%). Baseline risk factors are presented in Table 9. The independent Data Review Committee classified patients receiving CRESEMBA as primary therapy, or for invasive mold disease refractory to, or patients intolerant of other antifungal therapy. Table 9. Baseline Risk Factors in Mucorales Patients
Patients were treated with CRESEMBA intravenously or via oral administration at the recommended doses. Median treatment duration was 102 days for patients classified as primary, 33 days for refractory, and 85 days for intolerant [see Dosage and Administration (2.2)]. For patients with invasive mucormycosis, all-cause mortality through day 42 and success in overall response at the End-of-Treatment as assessed by the independent Data Review Committee is shown in Table 10. These results provide evidence that CRESEMBA is effective for the treatment for mucormycosis, in light of the natural history of untreated mucormycosis. However, the efficacy of CRESEMBA for the treatment for invasive mucormycosis has not been evaluated in concurrent, controlled clinical trials. Table 10. All-Cause Mortality and Overall Response Success in Mucorales Patients
15 REFERENCES • DePauw, B., Walsh, T.J., Donnelly, J.P., et al. (2008) Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Quadrature Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clinical Infectious Diseases 46:1813-1821. 16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Capsules CRESEMBA (isavuconazonium sulfate) capsules are available in aluminum blister packs. Each capsule contains 186 mg isavuconazonium sulfate (equivalent to 100 mg of isavuconazole). Capsules are opaque and elongated, and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a white cap imprinted with “766” in black ink. Store in original container to protect from moisture. Capsules are packaged in aluminum blister packs, seven (7) capsules per sheet with desiccant. (NDC 0469-0520-14) Injection CRESEMBA (isavuconazonium sulfate) for injection is supplied in a single-dose vial as white to yellow sterile lyophilized powder containing 372 mg isavuconazonium sulfate (equivalent to 200 mg isavuconazole). Individually packaged vials are available for intravenous administration. (NDC 0469-0420-99) 16.2 Storage and Handling Store CRESEMBA capsules at 20°C to 25°C (68°F to 77°F) in the original packaging to protect from moisture. Excursions are permitted from 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Store CRESEMBA for injection unreconstituted vials at 2° to 8°C (36° to 46°F) in a refrigerator. CRESEMBA is a single-dose vial of unpreserved sterile lyophile. Following reconstitution of the lyophile with water for injection USP, the reconstituted solution should be used immediately, or stored below 25°C for a maximum of 1 hour prior to preparation of the patient infusion solution. The prepared infusion solution should be kept for not more than 6 hours at room temperature [20°C to 25°C (68°F to 77°F)] or 24 hours at 2° to 8°C (36° to 46°F) prior to use. CRESEMBA for injection vials are for single-dose use only. 17 PATIENT COUNSELING INFORMATION Advise patients to read the FDA-approved patient labeling (Patient Information). Advise patients that CRESEMBA can be taken with or without food. Each capsule should be swallowed whole. Do not chew, crush, dissolve, or open the capsules. Advise patients to inform their physician if they are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of CRESEMBA. CRESEMBA can decrease or increase the plasma concentrations of other drugs. Advise patients to inform their physician if they are pregnant, plan to become pregnant, or are nursing. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8f7f73b8-586a-4df0-935f-fecd4696c16c | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||