|
【中文品名】磷苯妥英钠
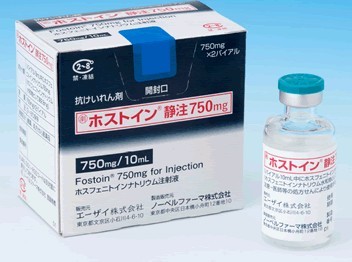
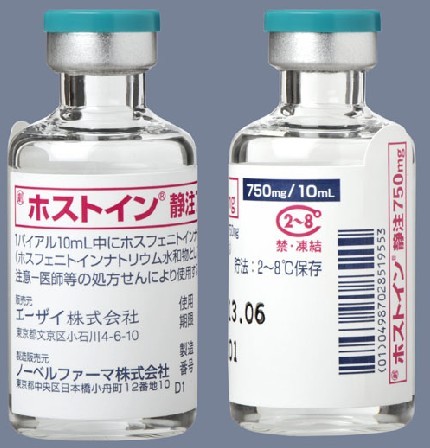
Cerebyx® label confusion, flawed dispensing practice, result in baby's death PROBLEM: Many hospitals have two standards of control for dispensing needed medications to patients. In the preferred standard, medication orders are screened for appropriateness by nurses and pharmacists before doses are prepared, checked and dispensed by a pharmacist in unit-dose form. Drugs dispensed by the pharmacy are subject to a system of redundant independent checks. In the other standard, with looser controls, medications are stored in patient care areas and removed as needed. No order screening takes place in the pharmacy. Doses are prepared, dispensed, and administered by the same person - usually a nurse - often from bulk containers. No system of independent redundant checks exists. This may increase the risk of a serious error, especially if drug product labeling is misunderstood. As reported in our August 27, 1997 issue (Cerebyx® dosing still a conundrum), the volume of the Cerebyx® (fosphenytoin) vial is listed in one position and the strength per milliliter ("50 mg PE/mL") is listed in another position. This makes it appear to some as though only 50 mg PE is in the vial. Cerebyx vials removed from active Pharmacy stock 5/1/98 Recently, a two-year-old child presented to the emergency room with seizures. Cerebyx 150 mg I.V. (100 mg PE) was ordered. Since most medications used in the ER were drawn from floor stock, pharmacy technicians delivered drugs upon verbal request. A technician misread the Cerebyx label and delivered 3 x 10 mL vials (1500 mg PE total). A nurse also misread the label, assuming that 50 mg was in each vial. She drew up 30 mL (1500 mg PE) instead of 150 mg of Cerebyx (100 mg PE) and handed it to another RN who administered the drug. Assuming the proper dose had been diluted, the second RN did not question the 30 mL volume. The patient died soon after injection. Excessive amounts of phenytoin were found in the child's blood. SAFE PRACTICE RECOMMENDATION: Medications and administration methods are far more complex and potentially dangerous than they were 25 years ago when many drug distribution systems were originally designed. In 1998, the need for patient safety dictates that hospital patient care units, especially critical care units, have medications dispensed only by the pharmacy upon receipt of a prescriber's medication order. Drugs should be prepared by pharmacy for each patient only after computer order screening to assure that the order is appropriate. Medications not dispensed by pharmacy should be authorized for storage in hospital areas only after due consideration is given to the degree of urgency as well as error potential. Drug distribution from pharmacy requires a pharmacist's check. Automated technology, satellite pharmacies or other methods should be used to assure appropriate order screening and dispensing. ISMP has previously contacted Parke-Davis about Cerebyx issues (August 1997; February, 1998) and we've offered our assistance again. So far they've informed us that the labeling is adequate and need not be changed. Errors with this product are fraught with danger. Consequently, Cerebyx is too dangerous for storage outside the pharmacy and should be removed from floor stock. If circumstances won't allow that, place it in bags with this note: "This 2 mL (or 10 mL) vial contains the equivalent of 100 mg (or 500 mg) of phenytoin (50 mg/mL)." Orders for fosphenytoin should not be accepted if "PE" is omitted. Promote dosing in terms of "PE." Some hospitals have placed restrictions on prescribing privileges for this drug. ホストイン静注750mg商標名 一般名: 化学名: 分子式: 分子量: 構造式: 性 状: 包装 ホストイン静注750mg 2バイアル 完整处方附件:http://www.info.pmda.go.jp/go/pack/1132401A1020_1_01/ 磷苯妥英钠(fosphenytoin disodium, Cerebyx)为美国WarnerLambert公司开发的新型抗癫痫药,1996年在美国首次上市,为苯妥英钠的前体药物,在体内可经磷酸酯酶迅速水解,全部转化为苯妥英钠而发挥作用。用于急性癫痫发作、癫痫持续状态和神经外科手术病人的癫痫发作及随之发生的并发症。 磷苯妥英钠的剂型为注射剂,规格750 mg∶10 mL或150 mg∶2 mL。 苯妥英钠可用于除小发作外各种癫痫发作的治疗,但作为急救药只能静脉滴注不能静脉注射,且注射部位疼痛、红肿,患者难以忍受。因此,其使用受到很大限制,很多国家已经停止使用苯妥英钠注射剂。而磷苯妥英钠最大的特点是可静脉注射给药治疗癫痫急性发作,依从性好,耐受性好,不良反应少,而且还可以肌内注射给药,适合用于难以口服给药的患者。副作用有眼球震颤、嗜睡、焦虑等。该药的临床使用为癫痫急症治疗提供了新途径。 |
磷苯妥英钠注射液Cerebyx(FOSPHENYTOIN SODIUM)简介:
【中文品名】磷苯妥英钠【药效类别】抗癫痫药>乙内酰脲衍生物类【通用药名】FOSPHENYTOIN SODIUM【别 名】Cerebyx【化学名称】 2,4-Imidazolidinedione, 5,5-diphenyl-3-[(phosphonooxy)methyl]-, d ... 责任编辑:admin |
最新文章更多推荐文章更多热点文章更多 |