|
部份奥扎格雷钠中文资料(仅供参考)
【中文品名】奥扎格雷钠
【药效类别】抗凝药>抗血小板聚集药
【通用药名】OZAGREL SODIUM
【别 名】Cataclot,Xanbon
【化学名称】 2-Propenoic acid, 3-[4-(1H-imidazol-1-ylmethyl)phenyl]-, (E)-, sodium salt
【CA登记号】[82571-53-7]
【结 构 式】
【分 子 式】C13H11N2NaO2
【分 子 量】250.22
【收录药典】
【开发单位】小野药品株式会社 (日本)
【首次上市】1987年,日本
【性 状】白色结晶或结晶性粉末,无臭,味酸或苦。易溶于水,稍易溶于甲醇,几乎不溶于无水乙醇、丙酮和乙醚,遇光稍不稳定,mp214~217℃。
【用 途】抗凝血药。可强烈抑制血栓素(TX)合成酶。用于蛛网膜下腔出血术后脑血管痉孪、伴随血管痉孪的脑缺血症状。
キサンボンS注射液20mg/キサンボンS注射液40mg
治疗类别名称
血栓素合成酶抑制剂
商標名
XANBON S injection 20mg
XANBON S injection 40mg
一般名
オザグレルナトリウム (Ozagrel Sodium)
化学名
Monosodium(2E)-3-[4-(1H-imidazol-1-ylmethyl)phenyl]prop-2-enoate
構造式
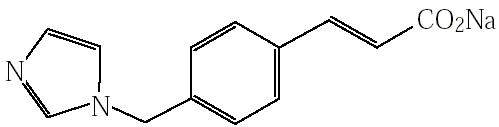
分子式
C13H11N2NaO2
分子量
250.23
*性状
本产品是一种白色结晶或结晶性粉末。此产品易溶于水,微溶于甲醇,和乙醇(99.5)几乎不溶。
操作注意事项
1.摇动安瓿,滴,在脖子上已经积累了液体

2.将安瓿主体,删除它扭曲上限。请不要在此时挤压身体。

3.您已在液体和注射器连接到安瓿前相同容量熏制更多的空气安瓿。

4.连接注射器的安瓿注射针无安装。在上面的安瓿,喷气管的注入后,拔出液体内容物。如果整个量没有Nukitore一次,请撤回反复泵送。
适应病症与用法用量
1.改善与脑血管痉挛有关的脑缺血症状,这种手术后蛛网膜下腔出血
成人,80毫克与电解质溶液或适当的量的糖溶液为奥扎格雷钠稀释的日剂量,持续超过24小时静脉内给药。给药开始蛛网膜下腔出血术后早期,理想的是两周连续施用。应当指出的是,增加或减少取决于患者的年龄和症状。
2.改善与脑血栓相关运动障碍(急性期)
成人,在单剂量80毫克与电解质溶液或适当的量的糖溶液为奥扎格雷钠稀释,每天早晨和傍晚进行的连续输注两次约两周的时间内2小时。应当指出的是,增加或减少取决于患者的年龄和症状
药效药理
1. 作用机序
本剤抑制血栓素A 2的选择性抑制血栓合酶,以促进生产前列环素,示出了具有改善两者的不平衡的血小板聚集抑制作用。另外,为了抑制脑血管痉挛和脑血流的减少,改善大脑的微循环紊乱和能量代谢异常,改善与这种手术后蛛网膜下腔出血相关的脑血管痉挛和脑缺血症状它,以及改善与脑血栓急性阶段有关的运动障碍。
2. 药理作用
(1) 奥扎格雷钠显示了血栓素A2(TXA2)人和兔血小板合成酶具有很强的抑制作用。另一方面,它不具有对环氧合酶,前列环素(PGI 2)合成酶,PGE 2异构酶和12-脂氧合酶的作用(体外)。
(2) TXA2,对生产PGI2的作用
1)当静脉内给药于健康成年人和脑血栓患者,TXA2产量显着地抑制,可以观察促进生产PGI2的。
2)连续静脉给药在大鼠脑动脉闭塞再灌注模型后梗阻(100μG/公斤/分钟)。然后,打压重新开放后,血浆PGI2/TXA2浓度比下降。
(3) 对血小板的影响
1)由花生四烯酸和胶原兔的富血小板血浆在10-5M-10-4M,另外,花生四烯酸在人类富含血小板的血浆,由胶原和ADP凝集,以及来自血小板的血清素释放以浓度依赖性方式的聚合抑制(体外)。
2)静脉内给予猫leptomingeal血管内皮损伤的模型(10毫克/公斤)。然后,抑制脑软脑膜动脉血小板血栓形成。
3)当兔子富血小板血浆通过加入10-4M的刺激与花生四烯酸,血小板环AMP的增加(体外)。
(4) 脑血管痉挛,脑血管直径和脑血流的影响
1)当直接注入自体血进入所述池连续静脉给药或在注入狗的蛛网膜下腔出血模型的大罐,以抑制痉挛和基底动脉局部脑血流量的降低。
2)连续静脉给药比两侧颈总动脉闭塞和自发性高血压大鼠重开闭模型之前(100μG/公斤/分钟)。然后,以抑制局部脑血流量减少。
3)静脉内给予猫leptomingeal血管内皮损伤的模型(10毫克/公斤)。然后,以延长leptomingeal动脉
4)当静脉内给药于脑血栓患者,HakuTadashinochi流量增大。
(5) 脑能量代谢的影响
1)连续静脉给药比两侧颈总动脉闭塞和自发性高血压大鼠重开闭模型之前(100μG/公斤/分钟)。然后,以抑制局部脑葡萄糖代谢下降。
2)静脉内给药在自发性高血压大鼠堵塞之前两侧总颈动脉闭塞和重开模型(30毫克/公斤)。然后,以抑制减少的增加和乳酸的大脑中的ATP的量。
(6) 脑栓塞的影响
1)静脉内给予兔子的脑梗塞模型花生四烯酸(0.3,1mg/kg)的连续输注。然后,它抑制脑梗塞的形成。
2)连续静脉给药相比在大鼠脑动脉闭塞 - 再灌注模型堵塞之前(100μG/千克/分钟)。然后,抑制脑梗塞的形成。
(7) 对运动功能障碍的影响
连续静脉给药在大鼠脑动脉闭塞 - 再灌注模型后阻塞(100μG/千克/分钟)。然后,以改善偏瘫的运动功能障碍,或类似物。
包装规格
S注射液
20毫克*10塑料安瓿
20毫克*50塑料安瓿

40毫克*10塑料安瓿
40毫克*50塑料安瓿

制造厂商
KISSEI药品有限公司
XANBON S injection(Ozagrel sodium)
XANBON S injection 20mg (キサンボンS注射液20mg[手术的蛛网膜下腔出血/脑血栓后使用])
Brand name : XANBON S injection 20mg [for use after surgery for subarachnoid hemorrhage/cerebral thrombosis]
Active ingredient: Ozagrel sodium
Dosage form: injection
Print on wrapping:
XANBON S injection 40mg(キサンボンS注射液40mg[手术的蛛网膜下腔出血/脑血栓后使用])
Brand name : XANBON S injection 40mg [for use after surgery for subarachnoid hemorrhage/cerebral thrombosis]
Active ingredient: Ozagrel sodium
Dosage form: injection
Print on wrapping:
Effects of this medicine
This medicine suppresses production of thromboxane A2 and promotes production of prostacyclin by selectively inhibiting thromboxane synthetase, leading to improvements in the imbalance between thromboxane A2 and prostacyclin, as well as to antiplatelet aggregation action.
It is usually used to improve symptoms of cerebral ischemia caused by constriction of blood vessels after surgery for subarachnoid hemorrhage.
Before using this medicine, be sure to tell your doctor and pharmacist
•If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have bleeding (hemorrhagic cerebral infarction, epidural bleeding, intra-cerebral bleeding, primary intraventricular bleeding), brain embolism, heart problems, cortical symptoms such as disturbance of consciousness or speech loss/agnosia or cardioembolic stroke.
•If you are pregnant or breastfeeding.
•If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Dosing schedule (How to take this medicine)
•Your dosing schedule prescribed by your doctor is <<to be written by a healthcare professional>>
•In general, inject intravenously over 24 hours.
•In general, inject every day for 2 consecutive weeks.
Precautions while taking this medicine
• Possible adverse reactions to this medicine
The most commonly reported adverse reactions include bleeding adverse reactions (epidural hematoma/intra-cerebral bleeding/gastrointestinal bleeding/subcutaneous bleeding, etc.), liver dysfunction, rash, fever, asthma (like) attack, itch, hives, erythema and blood pressure decreased. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
•headache, bloody stool, subcutaneous bleeding [bleeding]
•blood pressure decreased, respiratory distress, pharyngeal edema [shock, anaphylactoid symptoms]
•yellow discoloration of the skin and the white of eyes, general malaise, loss of appetite [liver dysfunction, jaundice]
•prolonged bleeding, gum bleeding, bruise [platelets decreased]
•fever, sore throat, general malaise [white blood cell decreased, granulocytopenia]
The above symptoms do not describe all the adverse reactions to this medicine. Consult with your doctor or pharmacist if you notice any symptoms of concern other than those listed above.
Kissei Pharmaceutical Co., Ltd.Injection
Revised: 8/2012
The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.
http://www.info.pmda.go.jp/go/pack/3999411H2038_1_05/
http://www.kissei.co.jp/di/cgi-bin2/regist.cgi?c=product-details&id=11
|

