|
抗真菌药泊沙康唑静脉剂型获批 1. INDICATIONS AND USAGE 1.1 Prophylaxis of Invasive Fungal Infection NOXAFIL Oral Suspension is indicated for prophylaxis of invasive Aspergillus and Candida infections in patients, 13 years of age and older, who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy. 1.2 Treatment of Oropharyngeal Candidiasis Including Oropharyngeal Candidiasis Refractory to Itraconazole and/or Fluconazole NOXAFIL is indicated for the treatment of oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole. 2. DOSAGE AND ADMINISTRATION 2.1 Dosage
Shake NOXAFIL Oral Suspension well before use.
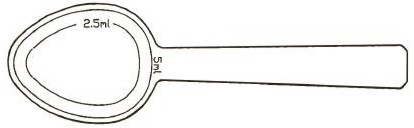
Figure 1: A measured dosing spoon is provided, marked for doses of 2.5 mL and 5 mL.
It is recommended that the spoon is rinsed with water after each administration and before storage. Each dose of NOXAFIL should be administered with a full meal or with a liquid nutritional supplement or an acidic carbonated beverage (e.g. ginger ale) in patients who cannot eat a full meal. To enhance the oral absorption of posaconazole and optimize plasma concentrations:
3. DOSAGE FORMS AND STRENGTHS NOXAFIL Oral Suspension is available in 4-ounce (123 mL) amber glass bottles with child-resistant closures (NDC 0085-1328-01) containing 105 mL of suspension (40 mg of posaconazole per mL). 4. CONTRAINDICATIONS 4.1 Hypersensitivity NOXAFIL is contraindicated in persons with known hypersensitivity to posaconazole, any component of NOXAFIL, or other azole antifungal agents. 4.2 Use With Sirolimus NOXAFIL is contraindicated with sirolimus. Concomitant administration of NOXAFIL with sirolimus increases the sirolimus blood concentrations by approximately 9 fold and can result in sirolimus toxicity [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. 4.3 QT Prolongation With Concomitant Use With CYP3A4 Substrates NOXAFIL is contraindicated with CYP3A4 substrates that prolong the QT interval. Concomitant administration of NOXAFIL with the CYP3A4 substrates, pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and rare occurrences of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)]. 4.4 Use With Simvastatin Concomitant administration of NOXAFIL with simvastatin increases the simvastatin plasma concentrations by approximately 10 fold. Increased plasma statin concentrations can be associated with rhabdomyolysis [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)]. 4.5 Use With Ergot Alkaloids Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism [see Drug Interactions (7.4)]. 5. WARNINGS AND PRECAUTIONS 5.1 Calcineurin-Inhibitor Drug Interactions Concomitant administration of NOXAFIL with cyclosporine or tacrolimus increases the whole blood trough concentrations of these calcineurin-inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Nephrotoxicity and leukoencephalopathy (including isolated deaths) have been reported in clinical efficacy studies in patients with elevated cyclosporine concentrations. Frequent monitoring of tacrolimus or cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus or cyclosporine dose adjusted accordingly. 5.2 Arrhythmias and QT Prolongation Some azoles, including posaconazole, have been associated with prolongation of the QT interval on the electrocardiogram. In addition, rare cases of torsades de pointes have been reported in patients taking posaconazole. Results from a multiple time-matched ECG analysis in healthy volunteers did not show any increase in the mean of the QTc interval. Multiple, time-matched ECGs collected over a 12-hour period were recorded at baseline and steady-state from 173 healthy male and female volunteers (18–85 years of age) administered posaconazole 400 mg BID with a high-fat meal. In this pooled analysis, the mean QTc (Fridericia) interval change from baseline was –5 msec following administration of the recommended clinical dose. A decrease in the QTc (F) interval (–3 msec) was also observed in a small number of subjects (n=16) administered placebo. The placebo-adjusted mean maximum QTc (F) interval change from baseline was <0 msec (–8 msec). No healthy subject administered posaconazole had a QTc (F) interval ≥500 msec or an increase ≥60 msec in their QTc (F) interval from baseline. Posaconazole should be administered with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs that are known to prolong the QTc interval and are metabolized through CYP3A4 [see Contraindications (4.3) and Drug Interactions (7.2)]. Rigorous attempts to correct potassium, magnesium, and calcium should be made before starting posaconazole. 5.3 Hepatic Toxicity Hepatic reactions (e.g., mild to moderate elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, and/or clinical hepatitis) have been reported in clinical trials. The elevations in liver function tests were generally reversible on discontinuation of therapy, and in some instances these tests normalized without drug interruption and rarely required drug discontinuation. Isolated cases of more severe hepatic reactions including cholestasis or hepatic failure including deaths have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with posaconazole. These severe hepatic reactions were seen primarily in subjects receiving the 800 mg daily (400 mg BID or 200 mg QID) in clinical trials. Liver function tests should be evaluated at the start of and during the course of posaconazole therapy. Patients who develop abnormal liver function tests during posaconazole therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver function tests and bilirubin). Discontinuation of posaconazole must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to posaconazole. 5.4 Use with Midazolam Concomitant administration of NOXAFIL with midazolam increases the midazolam plasma concentrations by approximately 5 fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Patients must be monitored closely for adverse effects associated with high plasma concentrations of midazolam and benzodiazepine receptor antagonists must be available to reverse these effects [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)]. 6. ADVERSE REACTIONS 6.1 Serious and Otherwise Important Adverse Reactions The following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling:
6.2 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of NOXAFIL cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of posaconazole therapy has been assessed in 1844 patients in clinical trials. This includes 605 patients in the active-controlled prophylaxis studies, 557 patients in the active-controlled OPC studies, 239 patients in refractory OPC studies, and 443 patients from other indications. This represents a heterogeneous population, including immunocompromised patients, e.g., patients with hematological malignancy, neutropenia post-chemotherapy, graft vs. host disease post hematopoietic stem cell transplant, and HIV infection, as well as non-neutropenic patients. This patient population was 71% male, had a mean age of 42 years (range 8–84 years, 6% of patients were ≥65 years of age and 1% was <18 years of age), and were 64% white, 16% Hispanic, and 36% non-white (including 14% black). Posaconazole therapy was given to 171 patients for ≥6 months, with 58 patients receiving posaconazole therapy for ≥12 months. Table 1 presents treatment-emergent adverse reactions observed at an incidence of >10% in posaconazole prophylaxis studies. Table 2 presents treatment-emergent adverse reactions observed at an incidence of at least 10% in the OPC/rOPC studies. Prophylaxis of Aspergillus and Candida: In the 2 randomized, comparative prophylaxis studies, the safety of posaconazole 200 mg three times a day was compared to fluconazole 400 mg once daily or itraconazole 200 mg twice a day in severely immunocompromised patients. The most frequently reported adverse reactions (>30%) in the prophylaxis clinical trials were fever, diarrhea and nausea. The most common adverse reactions leading to discontinuation of posaconazole in the prophylaxis studies were associated with GI disorders, specifically, nausea (2%), vomiting (2%), and hepatic enzymes increased (2%).
An additional 239 HIV-infected patients with refractory OPC received posaconazole in 2 non-comparative trials for refractory OPC (rOPC). Of these subjects, 149 received the 800-mg/day dose and the remainder received the ≤400-mg QD dose. In the OPC/rOPC studies, the most common adverse reactions were fever, diarrhea, nausea, headache, and vomiting. The most common adverse reactions that led to treatment discontinuation of posaconazole in the Controlled OPC Pool included respiratory insufficiency (1%) and pneumonia (1%). In the refractory OPC pool, the most common adverse reactions that led to treatment discontinuation of posaconazole were AIDS (7%) and respiratory insufficiency (3%).
Less Common Adverse Reactions: Clinically significant adverse reactions reported during clinical trials in prophylaxis, OPC/rOPC or other trials with posaconazole which occurred in less than 5% of patients are listed below:
Clinical Laboratory Values: In healthy volunteers and patients, elevation of liver function test values did not appear to be associated with higher plasma concentrations of posaconazole. The majority of abnormal liver function tests were minor, transient, and did not lead to discontinuation of therapy. For the prophylaxis studies, the number of patients with changes in liver function tests from Common Toxicity Criteria (CTC) Grade 0, 1, or 2 at baseline to Grade 3 or 4 during the study is presented in Table 3.
6.3 Postmarketing Experience No clinically significant postmarketing adverse reactions were identified that have not previously been reported during clinical trials experience. 7. DRUG INTERACTIONS Posaconazole is primarily metabolized via UDP glucuronidation and is a substrate of p-glycoprotein efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. Posaconazole is also a strong inhibitor of CYP3A4. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole [see Clinical Pharmacology (12.3)]. 7.1 Immunosuppressants Metabolized by CYP3A4 Sirolimus: Concomitant administration of posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9 fold and can result in sirolimus toxicity. Therefore, posaconazole is contraindicated with sirolimus [see Contraindications (4.2) and Clinical Pharmacology (12.3)]. Tacrolimus: Posaconazole has been shown to significantly increase the Cmax and AUC of tacrolimus. At initiation of posaconazole treatment, reduce the tacrolimus dose to approximately one-third of the original dose. Frequent monitoring of tacrolimus whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]. Cyclosporine: Posaconazole has been shown to increase cyclosporine whole blood concentrations in heart transplant patients upon initiation of posaconazole treatment. It is recommended to reduce cyclosporine dose to approximately three-fourths of the original dose upon initiation of posaconazole treatment. Frequent monitoring of cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the cyclosporine dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]. 7.2 CYP3A4 Substrates Concomitant administration of posaconazole with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and rare occurrences of torsades de pointes. Therefore, posaconazole is contraindicated with these drugs. [see Contraindications (4.3), and Warnings and Precautions (5.2)]. 7.3 HMG-CoA reductase Inhibitors (Statins) Metabolized Through CYP3A4 Concomitant administration of posaconazole with simvastatin increases the simvastatin plasma concentrations by approximately 10 fold. Therefore, posaconazole is contraindicated with HMG-CoA reductase inhibitor simvastatin [see Contraindications (4.4) and Clinical Pharmacology (12.3)]. Although not studied clinically with statins other than simvastatin, posaconazole may increase the plasma concentrations of statins that are metabolized by CYP3A4. Increased plasma statin concentrations can be associated with rhabdomyolysis. It is recommended that patients be monitored for adverse events and dose reduction of the statin be considered during co-administration with posaconazole. 7.4 Ergot Alkaloids Most of the ergot alkaloids are substrates of CYP3A4. Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism. Therefore, posaconazole is contraindicated with ergot alkaloids [see Contraindications (4.5)]. 7.5 Benzodiazepines Metabolized by CYP3A4 Concomitant administration of posaconazole with midazolam increases the midazolam plasma concentrations by approximately 5 fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Concomitant use of posaconazole and other benzodiazepines metabolized by CYP3A4 (e.g., alprazolam, triazolam) could result in increased plasma concentrations of these benzodiazepines. Patients must be monitored closely for adverse effects associated with high plasma concentrations of benzodiazepines metabolized by CYP3A4 and benzodiazepine receptor antagonists must be available to reverse these effects. [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)]. 7.6 Anti-HIV Drugs Efavirenz: Efavirenz induces UDP-glucuronidase and significantly decreases posaconazole plasma concentrations [see Clinical Pharmacology (12.3)]. It is recommended to avoid concomitant use of efavirenz with posaconazole unless the benefit outweighs the risks. Ritonavir and Atazanavir: Ritonavir and atazanavir are metabolized by CYP3A4 and posaconazole increases plasma concentrations of these drugs [see Clinical Pharmacology (12.3)]. Frequent monitoring of adverse effects and toxicity and dosage adjustments of ritonavir and atazanavir should be performed during co-administration with posaconazole. 7.7 Rifabutin Rifabutin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Rifabutin is also metabolized by CYP3A4. Therefore, Co-administration of rifabutin with posaconazole increases rifabutin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of posaconazole and rifabutin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring of breakthrough fungal infections as well as frequent monitoring of full blood counts and adverse reactions due to increased rifabutin plasma concentrations (e.g., uveitis, leukopenia) are recommended. 7.8 Phenytoin Phenytoin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Phenytoin is also metabolized by CYP3A4. Therefore, co-administration of phenytoin with posaconazole increases phenytoin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of posaconazole and phenytoin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring of breakthrough fungal infections is recommended and frequent monitoring of phenytoin concentrations should be performed while co-administered with posaconazole and dose reduction of phenytoin should be considered. 7.9 Gastric Acid Suppressors/Neutralizers Cimetidine (an H2-receptor antagonist) and esomeprazole (a proton pump inhibitor) decrease posaconazole plasma concentrations [see Clinical Pharmacology (12.3)]. It is recommended to avoid concomitant use of cimetidine and esomeprazole with posaconazole unless the benefit outweighs the risks. However, if concomitant administration is required, close monitoring of breakthrough fungal infections is recommended. No clinically relevant effects were observed when posaconazole is concomitantly used with antiacids and H2-receptor antagonists other than cimetidine. No dosage adjustment of posaconazole is required when posaconazole is concomitantly used with antacids and H2-receptor antagonists other than cimetidine. 7.10 Vinca Alkaloids Most of the vinca alkaloids are substrates of CYP3A4. Posaconazole may increase the plasma concentrations of vinca alkaloids (e.g., vincristine and vinblastine) which may lead to neurotoxicity. Therefore, it is recommended that dose adjustment of the vinca alkaloid be considered. 7.11 Calcium Channel Blockers Metabolized by CYP3A4 Posaconazole may increase the plasma concentrations of calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, diltiazem, nifedipine, nicardipine, felodipine). Frequent monitoring for adverse reactions and toxicity related to calcium channel blockers is recommended during co-administration. Dose reduction of calcium channel blockers may be needed. 7.12 Digoxin Increased plasma concentrations of digoxin have been reported in patients receiving digoxin and posaconazole. Therefore, monitoring of digoxin plasma concentrations is recommended during co-administration. 7.13 Gastrointestinal Motility Agents Metoclopramide decreases posaconazole plasma concentrations [see Clinical Pharmacology (12.3)]. If metoclopramide is concomitantly administered, it is recommended to closely monitor for breakthrough fungal infections. Loperamide does not affect posaconazole plasma concentrations [see Clinical Pharmacology (12.3)]. No dosage adjustment of posaconazole is required when loperamide and posaconazole are used concomitantly. 7.14 Glipizide Although no dosage adjustment of glipizide is required, it is recommended to monitor glucose concentrations when posaconazole and glipizide are concomitantly used. 8. USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. NOXAFIL should be used in pregnancy only if the potential benefit outweighs the potential risk to the fetus. Posaconazole has been shown to cause skeletal malformations (cranial malformations and missing ribs) in rats when given in doses ≥27 mg/kg (≥1.4 times the 400 mg BID regimen based on steady-state plasma concentrations of drug in healthy volunteers). The no-effect dose for malformations in rats was 9 mg/kg, which is 0.7 times the exposure achieved with the 400-mg BID regimen. No malformations were seen in rabbits at doses up to 80 mg/kg. In the rabbit, the no-effect dose was 20 mg/kg, while high doses of 40 mg/kg and 80 mg/kg, 2.9 or 5.2 times the exposure achieved with the 400-mg BID regimen, caused an increase in resorptions. In rabbits dosed at 80 mg/kg, a reduction in body weight gain of females and a reduction in litter size were seen. 8.3 Nursing Mothers Posaconazole is excreted in milk of lactating rats. It is not known whether NOXAFIL is excreted in human milk. Because of the potential for serious adverse reactions from NOXAFIL in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. 8.4 Pediatric Use The safety and effectiveness of posaconazole have been established in the age groups 13 to 17 years of age. The safety and effectiveness of posaconazole in pediatric patients below the age of 13 years have not been established. Use of posaconazole in these age groups is supported by evidence from adequate and well-controlled studies of posaconazole in adults with additional data. A total of 12 patients 13 to 17 years of age received 600 mg/day (200 mg three times a day) for prophylaxis of invasive fungal infections. The safety profile in these patients <18 years of age appears similar to the safety profile observed in adults. Based on pharmacokinetic data in 10 of these pediatric patients, the mean steady-state average posaconazole concentration (Cav) was similar between these patients and adults (≥18 years of age). A total of 16 patients 8 to 17 years of age were treated with 800 mg/day (400 mg twice a day or 200 mg four times a day) in a study for another indication. Based on pharmacokinetic data in 12 of these pediatric patients, the mean steady-state average posaconazole concentration (Cav) was similar between these patients and adults (≥18 years of age). In the prophylaxis studies, the mean steady-state posaconazole average concentration (Cav) was similar among ten adolescents (13 to 17 years of age) and adults (≥18 years of age). This is consistent with pharmacokinetic data from another study in which mean steady-state posaconazole Cav from 12 adolescent patients (8–17 years of age) was similar to that in the adults (≥18 years of age). 8.5 Geriatric Use Of the 605 patients randomized to posaconazole in the prophylaxis clinical trials, 63 (10%) were ≥65 years of age. In addition, 48 patients treated with ≥800-mg/day posaconazole in another indication were ≥65 years of age. No overall differences in safety were observed between the geriatric patients and younger patients; therefore, no dosage adjustment is recommended for geriatric patients. The pharmacokinetics of posaconazole are comparable in young and elderly subjects (≥65 years of age). No adjustment in the dosage of NOXAFIL is necessary in elderly patients (≥65 years of age) based on age. No overall differences in the pharmacokinetics and safety were observed between elderly and young subjects during clinical trials, but greater sensitivity of some older individuals cannot be ruled out. 8.6 Renal Insufficiency Following single-dose administration of 400 mg of the oral suspension, there was no significant effect of mild (CLcr: 50–80 mL/min/1.73m2, n=6) and moderate (CLcr: 20–49 mL/min/1.73m2, n=6) renal insufficiency on posaconazole pharmacokinetics; therefore, no dose adjustment is required in patients with mild to moderate renal impairment. In subjects with severe renal insufficiency (CLcr: <20 mL/min/1.73m2), the mean plasma exposure (AUC) was similar to that in patients with normal renal function (CLcr: >80 mL/min/1.73m2); however, the range of the AUC estimates was highly variable (CV=96%) in these subjects with severe renal insufficiency as compared to that in the other renal impairment groups (CV<40%). Due to the variability in exposure, patients with severe renal impairment should be monitored closely for breakthrough fungal infections [see Dosage and Administration (2)]. 8.7 Hepatic Insufficiency After a single oral dose of posaconazole 400 mg, the mean AUC was 43%, 27%, and 21% higher in subjects with mild (Child-Pugh Class A, N=6), moderate (Child-Pugh Class B, N=6), and severe (Child-Pugh Class C, N=6) hepatic insufficiency, respectively, compared to subjects with normal hepatic function (N=18). Compared to subjects with normal hepatic function, the mean Cmax was 1% higher, 40% higher, and 34% lower in subjects with mild, moderate, and severe hepatic insufficiency, respectively. The mean apparent oral clearance (CL/F) was reduced by 18%, 36%, and 28% in subjects with mild, moderate, and severe hepatic insufficiency, respectively, compared to subjects with normal hepatic function. The elimination half-life (t½) was 27 hours, 39 hours, 27 hours, and 43 hours in subjects with normal hepatic function and mild, moderate, and severe hepatic insufficiency, respectively. It is recommended that no dose adjustment of NOXAFIL is needed in patients with mild to severe hepatic insufficiency (Child-Pugh Class A, B, and C) [see Dosage and Administration (2), and Warnings and Precautions (5)]. 8.8 Gender The pharmacokinetics of posaconazole are comparable in men and women. No adjustment in the dosage of NOXAFIL is necessary based on gender. 8.9 Race The pharmacokinetic profile of posaconazole is not significantly affected by race. No adjustment in the dosage of NOXAFIL is necessary based on race. 10. OVERDOSAGE During the clinical trials, some patients received posaconazole up to 1600 mg/day with no adverse reactions noted that were different from the lower doses. In addition, accidental overdose was noted in one patient who took 1200 mg BID for 3 days. No related adverse reactions were noted by the investigator. Posaconazole is not removed by hemodialysis. 11. DESCRIPTION NOXAFIL is a triazole antifungal agent available as a suspension for oral administration. Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5- (2,4-difluorophenyl)tetrahydro-5- (1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[ (1S,2S)-1-ethyl-2-hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one with an empirical formula of C37H42F2N8O4 and a molecular weight of 700.8. The chemical structure is: Posaconazole is a white powder and is insoluble in water. NOXAFIL Oral Suspension is a white, cherry-flavored immediate-release suspension containing 40 mg of posaconazole per mL and the following inactive ingredients: polysorbate 80, simethicone, sodium benzoate, sodium citrate dihydrate, citric acid monohydrate, glycerin, xanthan gum, liquid glucose, titanium dioxide, artificial cherry flavor, and purified water. 12. CLINICAL PHARMACOLOGY 12.1 Mechanism of Action Posaconazole is a triazole antifungal agent [see Clinical pharmacology (12.4)]. 12.2 Pharmacodynamics Exposure Response Relationship: In clinical studies of immunocompromised patients, a wide range of plasma exposures to posaconazole was noted. A pharmacokinetic-pharmacodynamic analysis of patient data revealed an apparent association between average posaconazole concentrations (Cav) and prophylactic efficacy. A lower Cav may be associated with an increased risk of treatment failure [defined in the study as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or invasive fungal infections (IFI)]. To enhance the oral absorption of posaconazole and optimize plasma concentrations:
12.3 Pharmacokinetics Absorption: In clinical studies of immunocompromised patients, a wide range of plasma exposures to posaconazole was noted. A pharmacokinetic-pharmacodynamic analysis of patient data revealed an apparent association between average posaconazole concentrations (Cav) and prophylactic efficacy. A lower Cav may be associated with an increased risk of treatment failure [defined in the study as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or invasive fungal infections (IFI)]. Posaconazole is absorbed with a median Tmax of ~3 to 5 hours. Dose proportional increases in plasma exposure (AUC) to posaconazole were observed following single oral doses from 50 mg to 800 mg and following multiple-dose administration from 50 mg BID to 400 mg BID. No further increases in exposure were observed when the dose was increased from 400 mg BID to 600 mg BID in febrile neutropenic patients or those with refractory invasive fungal infections. Steady-state plasma concentrations are attained at 7 to 10 days following multiple-dose administration. Following single-dose administration of 200 mg, the mean AUC and Cmax of posaconazole are approximately 3 times higher when administered with a nonfat meal and approximately 4 times higher when administered with a high-fat meal (~50 gm fat) relative to the fasted state. Following single dose administration of 400 mg, the mean AUC and Cmax of posaconazole are approximately 3 times higher when administered with a liquid nutritional supplement (14 gm fat) relative to the fasted state (see Table 5). In order to assure attainment of adequate plasma concentrations, it is recommended to administer posaconazole with food or a nutritional supplement.
Posaconazole is highly protein bound (>98%), predominantly to albumin. Metabolism: Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose. Posaconazole is primarily metabolized via UDP glucuronidation (phase 2 enzymes) and is a substrate for p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. A summary of drugs studied clinically, which affect posaconazole concentrations, is provided in Table 8.
Excretion: Posaconazole is eliminated with a mean half-life (t½) of 35 hours (range: 20–66 hours) and a total body clearance (CL/F) of 32 L/hr. Posaconazole is predominantly eliminated in the feces (71% of the radiolabeled dose up to 120 hours) with the major component eliminated as parent drug (66% of the radiolabeled dose). Renal clearance is a minor elimination pathway, with 13% of the radiolabeled dose excreted in urine up to 120 hours (<0.2% of the radiolabeled dose is parent drug). 12.4 Microbiology Mechanism of Action: Posaconazole blocks the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of the enzyme lanosterol 14α-demethylase and accumulation of methylated sterol precursors. Activity in vitro and in vivo: Posaconazole has shown in vitro activity against Aspergillus fumigatus and Candida albicans, including Candida albicans isolates from patients refractory to itraconazole or fluconazole or both drugs [see Clinical Studies (14), Indications (1), and Dosage and Administration (2)]. In vitro susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) methods (M27-A2, M27-A, M38-A, M38-P). However, correlation between the results of susceptibility studies and clinical outcome has not been established. Posaconazole interpretive criteria/ breakpoints have not been established for any fungi. In immunocompetent and/or immunocompromised mice and rabbits with pulmonary or disseminated infection with A. fumigatus, posaconazole administered prophylactically was effective in prolonging survival and reducing mycological burden. Prophylactic posaconazole also prolonged survival of immunocompetent mice challenged with C. albicans or A. flavus. Drug Resistance: Clinical isolates of Candida albicans and Candida glabrata with decreases in posaconazole susceptibility were observed in oral swish samples taken during prophylaxis with posaconazole and fluconazole, suggesting a potential for development of resistance. These isolates also showed reduced susceptibility to other azoles, suggesting cross-resistance between azoles. The clinical significance of this finding is not known. 13. NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility No drug-related neoplasms were recorded in rats or mice treated with posaconazole for 2 years at doses higher than the clinical dose. In a 2-year carcinogenicity study, rats were given posaconazole orally at doses up to 20 mg/kg (females), or 30 mg/kg (males). These doses are equivalent to 3.9 or 3.5 times the exposure achieved with a 400-mg BID regimen, respectively, based on steady-state AUC in healthy volunteers administered a high-fat meal (400-mg BID regimen). In the mouse study, mice were treated at oral doses up to 60 mg/kg/day or 4.8 times the exposure achieved with a 400-mg BID regimen. Posaconazole was not genotoxic or clastogenic when evaluated in bacterial mutagenicity (Ames), a chromosome aberration study in human peripheral blood lymphocytes, a Chinese hamster ovary cell mutagenicity study, and a mouse bone marrow micronucleus study. Posaconazole had no effect on fertility of male rats at a dose up to 180 mg/kg (1.7 × the 400-mg BID regimen based on steady-state plasma concentrations in healthy volunteers) or female rats at a dose up to 45 mg/kg (2.2 × the 400-mg BID regimen). 13.2 Animal Toxicology and/or Pharmacology In immunocompetent and/or immunocompromised mice and rabbits with pulmonary or disseminated infection with A. fumigatus, posaconazole administered prophylactically was effective in prolonging survival and reducing mycological burden. Prophylactic posaconazole also prolonged survival of immunocompetent mice challenged with C. albicans or A. flavus [see Clinical Studies (14)]. 14. CLINICAL STUDIES 14.1 Prophylaxis of Aspergillus and Candida Infections Two randomized, controlled studies were conducted using posaconazole as prophylaxis for the prevention of invasive fungal infections (IFIs) among patients at high risk due to severely compromised immune systems. The first study (Study 1) was a randomized, double-blind trial that compared posaconazole oral suspension (200 mg three times a day) with fluconazole capsules (400 mg once daily) as prophylaxis against invasive fungal infections in allogeneic hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD). Efficacy of prophylaxis was evaluated using a composite endpoint of proven/probable IFIs, death, or treatment with systemic antifungal therapy (patients may have met more than one of these criteria). Study 1 assessed all patients while on study therapy plus 7 days and at 16 weeks post-randomization. The mean duration of therapy was comparable between the 2 treatment groups (80 days, posaconazole; 77 days, fluconazole). Table 10 contains the results from Study 1.
All-cause mortality was similar at 16 weeks for both treatment arms in Study 1 [POS 58/301 (19%) vs. FLU 59/299 (20%)]; all-cause mortality was lower at 100 days for posaconazole-treated patients in Study 2 [POS 44/304 (14%) vs. FLU/ITZ 64/298 (21%)]. Both studies demonstrated substantially fewer breakthrough infections caused by Aspergillus species in patients receiving posaconazole prophylaxis when compared to patients receiving fluconazole or itraconazole. 14.2 Treatment of Oropharyngeal Candidiasis Study 3 was a randomized, controlled, evaluator-blinded study in HIV-infected patients with oropharyngeal candidiasis. Patients were treated with posaconazole or fluconazole oral suspension (both posaconazole and fluconazole were given as follows: 100 mg twice a day for 1 day followed by 100 mg once a day for 13 days). Clinical and mycological outcomes were assessed after 14 days of treatment and at 4 weeks after the end of treatment. Patients who received at least 1 dose of study medication and had a positive oral swish culture of Candida species at baseline were included in the analyses (Table 12). The majority of the subjects had C. albicans as the baseline pathogen. Clinical success at Day 14 (complete or partial resolution of all ulcers and/or plaques and symptoms) and clinical relapse rates (recurrence of signs or symptoms after initial cure or improvement) 4 weeks after the end of treatment were similar between the treatment arms (Table 12). Mycologic eradication rates (absence of colony forming units in quantitative culture at the end of therapy, Day 14), as well as mycologic relapse rates (4 weeks after the end of treatment) were also similar between the treatment arms (see Table 12).
Mycologic response rates, using a criterion for success as a post-treatment quantitative culture with ≤20 colony forming units (CFU/mL) were also similar between the two groups (posaconazole 68.0%, fluconazole 68.1%). The clinical significance of this finding is unknown. 14.3 Treatment of Oropharyngeal Candidiasis Refractory to Treatment With Fluconazole or Itraconazole Study 4 was a noncomparative study of posaconazole oral suspension in HIV-infected subjects with OPC that was refractory to treatment with fluconazole or itraconazole. An episode of OPC was considered refractory if there was failure to improve or worsening of OPC after a standard course of therapy with fluconazole ≥ 100 mg/day for at least 10 consecutive days or itraconazole 200 mg/day for at least 10 consecutive days and treatment with either fluconazole or itraconazole had not been discontinued for more than 14 days prior to treatment with posaconazole. Of the 199 subjects enrolled in this study, 89 subjects met these strict criteria for refractory infection. Forty-five subjects with refractory OPC were treated with posaconazole 400 mg BID for 3 days, followed by 400 mg QD for 25 days with an option for further treatment during a 3-month maintenance period. Following a dosing amendment, a further 44 subjects were treated with posaconazole 400 mg BID for 28 days. The efficacy of posaconazole was assessed by the clinical success (cure or improvement) rate after 4 weeks of treatment. The clinical success rate was 74.2% (66/89). The clinical success rates for both the original and the amended dosing regimens were similar (73.3% and 75.0%, respectively). 16. HOW SUPPLIED/STORAGE AND HANDLING Supplied with each bottle is a plastic dosing spoon calibrated for measuring 2.5-mL and 5-mL doses. Store at 25°C (77°F); excursions permitted to 15°–30°C (59°–86°F). DO NOT FREEZE. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||