日本药品和食品卫生理事会的一个评审委员会推荐批准大日本住友公司的Miripla(miriplatin)用于肝细胞癌(HCC)的治疗。同时获得肯定推荐的有ToaYakushin公司的万古霉素(vancomycin)眼科软膏配方,用于范围广泛的眼科感染。这2个产品最终要在今后几个月内经过厚生劳动省(MHLw)的批准,而miriplatin的批准将是世界范围内的第一次。
--------------------------------------------------------------------------------
October 16, 2009 -- Dainippon Sumitomo Pharma Co., Ltd. (Head Office:
“MIRIPLA®” is first suspended in an oily lymphographic agent and then administered through hepatic artery into hepatocellular carcinoma. As such an oily lymphographic agent, the Company has “MIRIPLA® suspension vehicle 4 mL” (generic name: iodine addition products of the ethylesters of the fatty acids obtained from poppyseed oil), which is approved for suspending MIRIPLA®. The manufacturing and marketing approval for this suspension vehicle was obtained on August 20, 2009 from the competent Ministry.
“Lipiodolization” or “Chemo-lipiodlization” is one of the standard methods for treating hepatocellular carcinoma , where an anticancer drug is suspended in an oily lymphographic agent (iodine addition products of the ethylesters of the fatty acids obtained from poppyseed oil, Lipiodol) and then administered into hepatic artery. The Company has carried out a research program to discover an anticancer drug suitable for this treatment and succeeded in development of a lipophilic platinum complex, miriplatin which has high affinity to Lipiodol.
MIRIPLA®has a high suspensibility in “MIRIPLA® suspension vehicle 4 mL “. Some of the characteristics of MIRIPLA®are: it accumulates and stays in a tumor after the administration into hepatic artery, platinum component is released gradually over a long duration and yet exposure to entire body is minor. In clinical tests on hepatocellular carcinoma, satisfactory anti-tumor effects were confirmed not only on patients of initial treatment but also on patients who relapsed after treatment such as hepatic resection. Some side effects were observed, but they were regarded as those generally observed under chemo-lipiodolization therapy, and they were thought to be tolerable at medical institutions familiarized with this treatment. There was no adverse event on vessel disorder in hepatic artery related to this drug.
The Company has an intention to launch both “MIRIPLA® for intra-arterial injection 70 mg” and “MIRIPLA® suspension vehicle 4 mL” after they are listed on the national health insurance drug price standard. As a result of launching of MIRIPLA®, the Company expects to increase the line-up of products for the liver diseases, which includes Sumiferon®, a natural interferon-alpha product, and to further contribute to the total care of liver diseases.
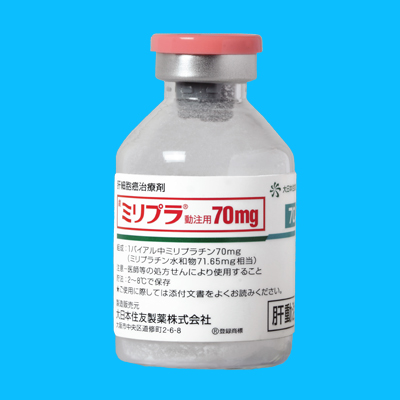
Miripla(miriplatin,ミリプラ)
肝癌への肝動脈塞栓療法に使用する
脂溶性白金製剤「ミリプラ」が2010年1月20日付で発売されました。
肝癌に限らず現在の治療で何とか凌いでいけば、
次々に新しい手段が利用できる時代になっています。
--------------------------------------------------------------
原产地英文商品名:
Miripla(ミリプラ)70mg/vial
原产地英文药品名:
miriplatin
中文参考商品译名:
Miripla(ミリプラ)70毫克/瓶
中文参考药品译名:
miriplatin
生产厂家中文参考译名:
大日本住友製薬
生产厂家英文名:
Dainihon Sumitomo
---------------------------------------------------------------