制造商:
第一三共
药理分类:
抗高血压(血管紧张素2受体阻滞剂+二氢吡啶钙通道阻滞剂+噻嗪类利尿剂)。
活性成分(补):
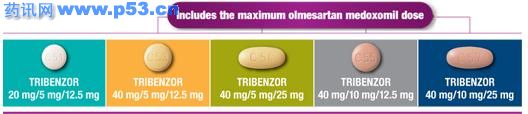
奥美沙坦酯20毫克,氨氯地平(如地平)5毫克,氢氯噻嗪(氢氯噻嗪)12.5mg;标签。
另外:
TRIBENZOR 40/5/12.5mg
奥美沙坦酯40毫克,氨氯地平(如地平)毫克,氢氯噻嗪(氢氯噻嗪)12.5mg;标签。
TRIBENZOR 40/5/25mg
奥美沙坦酯40毫克,氨氯地平(如地平),毫克,氢氯噻嗪(氢氯噻嗪)为25mg;标签。
TRIBENZOR 40/10/12.5mg
奥美沙坦酯40毫克,氨氯地平(如地平)10毫克,氢氯噻嗪(氢氯噻嗪)12.5mg;标签。
TRIBENZOR 40/10/25mg
奥美沙坦酯40毫克,氨氯地平(如地平)10毫克,氢氯噻嗪(氢氯噻嗪)25毫克;标签。
指示(补):
高血压。不适用于初步治疗。
药理作用:
Tribenzor结合抗高血压类药物制剂三个代表:血管紧张素2受体阻滞剂(ARB),钙通道阻滞剂(CCB)的,和利尿剂。这表明治疗高血压是没有充分对其中任何两个最佳类药物剂量的控制,也可能对个体滴定元件取代。
奥美,一种ARB,阻断血管紧张素Ⅱ结合的血管平滑肌AT1受体,从而减少血管收缩的血管紧张素Ⅱ介导的。氨氯地平是建设银行,抑制二氢钙离子进入血管平滑肌和心肌跨膜内流。噻嗪类利尿剂氢氯噻嗪是可以增加的钠和氯离子的排泄。
临床试验:
的研究进行了评价2492病人在高血压治疗中的Tribenzor疗效。患者随机接受治疗的三双组合之一2-4周,然后他们随机接受继续治疗的双重或接收三联疗法。 8周后,三联疗法产生收缩压和舒张压相比,在三个双结合疗法每个压力较大幅度的削减。
法律分类:
接收
成人:
每天一粒。滴定在2个星期的时间间隔,最大一40/10/25mg片剂每日。替代疗法:可取代单独滴定组成部分。 Add-on/switch治疗:可用于提供额外的BP没有充分的最高控制容忍,标示或通常的任何两个降低患者降压剂类:ARB类药物,钙通道阻滞剂和利尿剂。
儿童:
不推荐。
禁忌(补):
无尿。过敏磺胺类药物。
警告/注意事项:
严重肾功能损害(CrCl为≤30mL/min):不推荐。肾动脉狭窄。如果挂起肾功能不全的进展。正确盐/容衰竭之前开始治疗。严重肝功能障碍。严重心衰或阻塞性冠状动脉疾病。重度主动脉瓣狭窄。糖尿病。 Postsympathec到我的。系统性红斑狼疮。痛风。监测电解质。长者(“75岁)。妊娠(在第一孕期Cat.C; Cat.D在第二及第三孕期,避免)。哺乳母亲:不推荐。
互动(补):
洋地黄,锂毒性。调整反迪abetic,antigout药物。促肾上腺皮质激素,两性霉素B,皮质类固醇增加低钾血症的风险。体位性低血压potentiated用酒精,中枢神经系统抑制剂。拮抗的NSAIDs。电位- tiates其他降压药。非去极化肌松药,会增强。可拮抗肾上腺素。五月至甲状旁腺干扰试验。
不良反应(补):
头晕,周边水肿,头痛,疲劳,鼻咽炎,肌肉痉挛,胃肠不适,URI时,尿路感染,关节肿胀,高尿酸血症,体位性低血压,电解质紊乱。
如何提供:
制表- 30,90
最后更新:
2010年10月14日
【原产地英文商品名】TRIBENZOR (40/5/25)mg/tab 30tabs/bottle
【原产地英文药品名】OLMESARTAN MEDOXOMIL/AMLODIPINE BESYLATE/HYDROCHLOROTHIAZIDE
【中文参考商品译名】
注:以下产品是不同规格和不同的价格,购买时请以电话咨询为准!
TRIBENZOR (40/5/25)毫克/片 30片/瓶
TRIBENZOR (40/5/12.5)毫克/片 30片/瓶
TRIBENZOR (40/10/25)毫克/片 30片/瓶
TRIBENZOR (40/10/12.5)毫克/片 30片/瓶
TRIBENZOR (20/5/12.5)毫克/片 30片/瓶
【中文参考药品译名】奥美沙坦酯/苯磺酸氨氯地平/氢氯噻嗪
【生产厂家中文参考译名】第一三共
【生产厂家英文名】DAIICHI SANKYO
TRIBENZOR 20/5/12.5mg
Manufacturer:
Daiichi Sankyo
Pharmacological Class:
Antihypertensive (angiotensin 2 receptor blocker + dihydropyridine calcium channel blocker + thiazide diuretic).
Active Ingredient(s):
Olmesartan medoxomil 20mg, amlodipine (as besylate) 5mg, hydrochlorothiazide (HCTZ) 12.5mg; tabs.
Also:
TRIBENZOR 40/5/12.5mg
Olmesartan medoxomil 40mg, amlodipine (as besylate) 5mg, hydrochlorothiazide (HCTZ) 12.5mg; tabs.
TRIBENZOR 40/5/25mg
Olmesartan medoxomil 40mg, amlodipine (as besylate) 5mg, hydrochlorothiazide (HCTZ) 25mg; tabs.
TRIBENZOR 40/10/12.5mg
Olmesartan medoxomil 40mg, amlodipine (as besylate) 10mg, hydrochlorothiazide (HCTZ) 12.5mg; tabs.
TRIBENZOR 40/10/25mg
Olmesartan medoxomil 40mg, amlodipine (as besylate) 10mg, hydrochlorothiazide (HCTZ) 25mg; tabs.
Indication(s):
Hypertension. Not for initial therapy.
Pharmacology:
Tribenzor combines antihypertensive agents representative of three drug classes: an angiotensin 2 receptor blocker (ARB), a calcium channel blocker (CCB), and a diuretic. It is indicated to treat hypertension that is not adequately controlled on optimal doses of any two of these drug classes, or it may be substituted for the individually-titrated components.
Olmesartan, an ARB, blocks the binding of angiotensin II to the AT1 receptor in vascular smooth muscle, thereby reducing vasoconstriction mediated by angiotensin II. Amlodipine is a dihydropyridine CCB that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Hydrochlorothiazide is a thiazide diuretic that increases the excretion of sodium and chloride ions.
Clinical Trials:
A study was conducted in 2492 patients to evaluate the efficacy of Tribenzor in the treatment of hypertension. Patients were randomized to receive one of the three dual therapy combinations for 2–4 weeks, then they were randomized to either continue on the dual therapy or to receive triple therapy. After 8 weeks, triple therapy produced greater reductions in both systolic and diastolic blood pressures compared to each of the three dual combination therapies.
Legal Classification:
Rx
Adults:
One tablet daily. Titrate at 2-week intervals; max one 40/10/25mg tablet daily. Replacement therapy: may be substituted for individually titrated components. Add-on/switch therapy: may be used to provide additional BP lowering for patients not adequately controlled on max tolerated, labeled or usual doses of any two antihypertensive classes: ARBs, calcium channel blockers, and diuretics.
Children:
Not recommended.
Contraindication(s):
Anuria. Sulfonamide allergy.
Warnings/Precautions:
Severe renal impairment (CrCl ≤30mL/min): not recommended. Renal artery stenosis. Suspend if renal dysfunction progresses. Correct salt/volume depletion prior to starting therapy. Severe hepatic dysfunction. Severe CHF or obstructive coronary disease. Severe aortic stenosis. Diabetes. Postsympathectomy. SLE. Gout. Monitor electrolytes. Elderly (>75 years). Pregnancy (Cat.C in 1st trimester; Cat.D in 2nd and 3rd trimesters; avoid). Nursing mothers: not recommended.
Interaction(s):
Digitalis, lithium toxicity. Adjust antidiabetic, antigout medications. ACTH, amphotericin B, corticosteroids increase hypokalemia risk. Orthostatic hypotension potentiated by alcohol, CNS depressants. Antagonized by NSAIDs. Potentiates other antihypertensives. May potentiate nondepolarizing muscle relaxants. May antagonize norepinephrine. May interfere with parathyroid tests.
Adverse Reaction(s):
Dizziness, peripheral edema, headache, fatigue, nasopharyngitis, muscle spasms, GI upset, URI, UTI, joint swelling, hyperuricemia, orthostatic hypotension, electrolyte disturbances.
How Supplied:
Tabs—30, 90