|
英文药名: Dilantin Infatabs (Phenytoin acid form Chewable Tablets) 中文药名: 苯妥英钠(苯妥酸咀嚼片) 生产品牌药厂家: Pfizer 药品名称 别名: 大仑丁, 苯妥英钠,奇非宁, 大伦丁, 二苯乙内酰脲, 二苯乙内酰胺钠 本品为抗癫癎药、抗心律失常药。治疗剂量不引起镇静催眠作用, 口服吸收较慢,85~90%由小肠吸收,吸收率个体差异大,受食物影响。新生儿吸收甚差。口服生物利用度约为79%,分布于细胞内外液,细胞内可能多于细胞外,表观分布容积为0.6L/kg。血浆蛋白结合率为88~92%,主要与白蛋白结合, 在脑组织内蛋白结合可能还高。口服后4~12小时血药浓度达峰值。主要在肝脏代谢,代谢物无药理活性,其中主要为羟基苯妥英(约占50~70%),此代谢存在遗传多态性和人种差异。存在肠肝循环,主要经肾排泄,碱性尿排泄较快。T1/2为7~42小时,长期服用苯妥英钠的患者,T1/2 可为15~95小时,甚至更长。应用一定剂量药物后肝代谢(羟化)能力达饱和,此时即使增加很小剂量,血药浓度非线性急剧增加,有中毒危险,要监测血药浓度。有效血药浓度为10~20mg/L,每日口服300 mg,7~10日可达稳态浓度。血药浓度超过20mg/L时易产生毒性反应,出现眼球震颤;超过30mg/L时,出现共济失调;超过40mg/L时往往出现严重毒性作用。能通过胎盘,能分泌入乳汁。 适用于治疗全身强直-阵孪性发作、复杂部分性发作(精神运动性发作、颞叶癫癎)、单纯部分性发作(局限性发作)和癫癎持续状态。 抗癫癎 本品副作用小,常见齿龈增生,儿童发生率高,应加强口腔卫生和按摩齿龈。长期服用后或血药浓度达30μg/ml可能引起恶心,呕吐甚至胃炎,饭后服用可减轻。神经系统不良反应与剂量相关,常见眩晕、头痛,严重时可引起眼球震颤、共济失调、语言不清和意识模糊,调整剂量或停药可消失; 较少见的神经系统不良反应有头晕、失眠、一过性神经质、颤搐、舞蹈症、肌张力不全、震颤、扑翼样震颤等。可影响造血系统,致粒细胞和血小板减少,罕见再障;常见巨幼红细胞性贫血,可用叶酸加维生素B12防治。可引起过敏反应,常见皮疹伴高烧,罕见严重皮肤反应,如剥脱性皮炎,多形糜烂性红斑,系统性红斑狼疮和致死性肝坏死、淋巴系统何杰金病等。 禁用:对乙内酰脲类药有过敏史或阿斯综合征、Ⅱ~Ⅲ度房室阻滞,窦房结阻滞、窦性心动过缓等心功能损害者。 1.对乙内酰脲类中一种药过敏者,对本品也过敏。 本品能通过胎盘,可能致畸,但有认为癫癎发作控制不佳致畸的危险性大于用药的危险性,应权衡利弊。凡用本品能控制发作的患者,孕期应继续服用,并保持有效血浓,分娩后再重新调整。产前一个月应补充维生素K,产后立即给新生儿注射维生素K减少出血危险。本品可分泌入乳汁,一般主张服用苯妥英的母亲避免母乳喂养。 小儿由于分布容积与消除半衰期随年龄而变化,因此应经常作血药浓度测定。 新生儿或婴儿期对本品的药动学较特殊,临床对中毒症状评定有困难,一般不首先采用。学龄前儿童肝脏代谢强,需多次监测血药浓度以决定用药次数和用量。 老年人慢性低蛋白血症的发生率高,治疗上合并用药又较多,药物彼此相互作用复杂,应用本品时须慎重,用量应偏低,并经常监测血药浓度。 1. 长期应用对乙酰氨基酚患者应用本品可增加肝脏中毒的危险,并且疗效降低。 可出现视力模糊或复视,笨拙或行走不稳和步态蹒跚、精神紊乱,严重的眩晕或嗜睡,幻觉、恶心、语言不清。治疗:无解毒药,仅对症治疗和支持疗法,催吐,洗胃,给氧,升压,辅助呼吸,血液透析。 苯妥英钠胶囊/片 30mg, 100mg; DILANTIN INFATABS - phenytoin tablet, chewable ---------- INFATABS® NOT FOR ONCE-A-DAY DOSING DESCRIPTIONDilantin is an antiepileptic drug. Dilantin (phenytoin) is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is 5,5-diphenyl-2,4-imidazolidinedione, having the following structural formula: 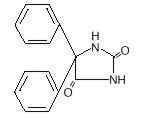 Each Dilantin Infatab, for oral administration, contains 50 mg phenytoin, USP. Also contains: D&C yellow No. 10, Al lake; FD&C yellow No. 6, Al lake; flavor; saccharin sodium, USP; sucrose, NF; talc, USP; and other ingredients. CLINICAL PHARMACOLOGYPhenytoin is an antiepileptic drug which can be useful in the treatment of epilepsy. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of posttetanic potentiation at synapses. Loss of posttetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Phenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of tonic-clonic (grand mal) seizures. Clinical studies using Dilantin Infatabs have shown an average plasma half-life of 14 hours with a range of 7 to 29 hours. Steady-state therapeutic levels are achieved at least 7 to 10 days (5–7 half-lives) after initiation of therapy with recommended doses of 300 mg/day. When serum level determinations are necessary, they should be obtained at least 5–7 half-lives after treatment initiation, dosage change, or addition or subtraction of another drug to the regimen so that equilibrium or steady-state will have been achieved. Trough levels provide information about clinically effective serum level range and confirm patient compliance and are obtained just prior to the patient's next scheduled dose. Peak levels indicate an individual's threshold for emergence of dose-related side effects and are obtained at the time of expected peak concentration. For Dilantin Infatabs, peak levels occur 1½ –3 hours after administration. Optimum control without clinical signs of toxicity occurs more often with serum levels between 10 and 20 mcg/mL, although some mild cases of tonic-clonic (grand mal) epilepsy may be controlled with lower serum levels of phenytoin. In most patients maintained at a steady dosage, stable phenytoin serum levels are achieved. There may be wide interpatient variability in phenytoin serum levels with equivalent dosages. Patients with unusually low levels may be noncompliant or hypermetabolizers of phenytoin. Unusually high levels result from liver disease, congenital enzyme deficiency, or drug interactions which result in metabolic interference. The patient with large variations in phenytoin plasma levels, despite standard doses, presents a difficult clinical problem. Serum level determinations in such patients may be particularly helpful. As phenytoin is highly protein bound, free phenytoin levels may be altered in patients whose protein binding characteristics differ from normal. Most of the drug is excreted in the bile as inactive metabolites which are then reabsorbed from the intestinal tract and excreted in the urine. Urinary excretion of phenytoin and its metabolites occurs partly with glomerular filtration but, more importantly, by tubular secretion. Because phenytoin is hydroxylated in the liver by an enzyme system which is saturable at high plasma levels, small incremental doses may increase the half-life and produce very substantial increases in serum levels, when these are in the upper range. The steady-state level may be disproportionately increased, with resultant intoxication, from an increase in dosage of 10% or more. Clinical studies show that chewed and unchewed Dilantin Infatabs are bioequivalent, yield approximately equivalent plasma levels, and are more rapidly absorbed than 100-mg Dilantin Kapseals®. INDICATIONS AND USAGEDilantin Infatabs (Phenytoin Tablets, USP) are indicated for the control of generalized tonic-clonic (grand mal) and complex partial (psychomotor, temporal lobe) seizures and prevention and treatment of seizures occurring during or following neurosurgery. Phenytoin serum level determinations may be necessary for optimal dosage adjustments (see DOSAGE AND ADMINISTRATION and CLINICAL PHARMACOLOGY sections). CONTRAINDICATIONSPhenytoin is contraindicated in those patients who are hypersensitive to phenytoin or other hydantoins. WARNINGSEffects of Abrupt WithdrawalAbrupt withdrawal of phenytoin in epileptic patients may precipitate status epilepticus. When, in the judgment of the clinician, the need for dosage reduction, discontinuation, or substitution of alternative antiepileptic medication arises, this should be done gradually. However, in the event of an allergic or hypersensitivity reaction, rapid substitution of alternative therapy may be necessary. In this case, alternative therapy should be an antiepileptic drug not belonging to the hydantoin chemical class. Suicidal Behavior and IdeationAntiepileptic drugs (AEDs), including Dilantin Infatabs, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide. The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed. The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5–100 years) in the clinical trials analyzed. Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications. Anyone considering prescribing Dilantin Infatabs or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated. Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers. LymphadenopathyThere have been a number of reports suggesting a relationship between phenytoin and the development of lymphadenopathy (local or generalized) including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin's disease. Although a cause and effect relationship has not been established, the occurrence of lymphadenopathy indicates the need to differentiate such a condition from other types of lymph node pathology. Lymph node involvement may occur with or without symptoms and signs resembling serum sickness, e.g., fever, rash, and liver involvement. In all cases of lymphadenopathy, follow-up observation for an extended period is indicated and every effort should be made to achieve seizure control using alternative antiepileptic drugs. Effects of Alcohol Use on Phenytoin Serum LevelsAcute alcoholic intake may increase phenytoin serum levels while chronic alcoholic use may decrease serum levels. Exacerbation of PorphyriaIn view of isolated reports associating phenytoin with exacerbation of porphyria, caution should be exercised in using this medication in patients suffering from this disease. Usage in PregnancyClinical
PreclinicalIncreased resorption and malformation rates have been reported following administration of phenytoin doses of 75 mg/kg or higher (approximately 120% of the maximum human loading dose or higher on a mg/m2 basis) to pregnant rabbits. Skin reactionsDilantin can cause rare, serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs hypersensitivity such as itching, and should seek medical advice from their physician immediately when observing any indicative signs or symptoms. The physician should advise the patient to discontinue treatment if the rash appears (see WARNINGS section regarding drug discontinuation). If the rash is of a milder type (measles-like or scarlatiniform), therapy may be resumed after the rash has completely disappeared. If the rash recurs upon reinstitution of therapy, further Dilantin medication is contraindicated. Published literature has suggested that there may be an increased, although still rare, risk of hypersensitivity reactions, including skin rash, SJS, TEN, hepatotoxicity, and Anticonvulsant Hypersensitivity Syndrome in black patients. Studies in patients of Chinese ancestry have found a strong association between the risk of developing SJS/TEN and the presence of HLA-B*1502, an inherited allelic variant of the HLA B gene, in patients using another anticonvulsive drug. Limited evidence suggests that HLA-B*1502 may be a risk factor for the development of SJS/TEN in patients of Asian ancestry taking drugs associated with SJS/TEN, including phenytoin. Consideration should be given to avoiding use of drugs associated with SJS/TEN, including Dilantin, in HLA-B*1502 positive patients when alternative therapies are otherwise equally available. Anticonvulsant Hypersensitivity SyndromeAnticonvulsant Hypersensitivity Syndrome (AHS) is a rare drug induced, multiorgan syndrome which is potentially fatal and occurs in some patients taking anticonvulsant medication. It is characterized by fever, rash, lymphadenopathy, and other multiorgan pathologies, often hepatic. The mechanism is unknown. The interval between first drug exposure and symptoms is usually 2–4 weeks but has been reported in individuals receiving anticonvulsants for 3 or more months. Although up to 1 in 5 patients on Dilantin may develop cutaneous eruptions, only a small proportion will progress to AHS. Patients at higher risk for developing AHS include black patients, patients who have a family history of or who have experienced this syndrome in the past, and immuno-suppressed patients. The syndrome is more severe in previously sensitized individuals. If a patient is diagnosed with AHS, discontinue the Dilantin and provide appropriate supportive measures. PRECAUTIONSGeneralThe liver is the chief site of biotransformation of phenytoin; patients with impaired liver function, elderly patients, or those who are gravely ill may show early signs of toxicity. A small percentage of individuals who have been treated with phenytoin have been shown to metabolize the drug slowly. Slow metabolism may be due to limited enzyme availability and lack of induction; it appears to be genetically determined. Published literature has suggested that there may be an increased, although still rare, risk of hypersensitivity reactions, including skin rash, SJS, TEN, hepatotoxicity, and Anticonvulsant Hypersensitivity Syndrome in black patients. (See WARNINGS section). Phenytoin should be discontinued if a skin rash appears (see WARNINGS section regarding drug discontinuation). If the rash is exfoliative, purpuric, or bullous or if lupus erythematosus, Stevens-Johnson syndrome, or toxic epidermal necrolysis is suspected, use of this drug should not be resumed and alternative therapy should be considered. (See ADVERSE REACTIONS.) If the rash is of a milder type (measles-like or scarlatiniform), therapy may be resumed after the rash has completely disappeared. If the rash recurs upon reinstitution of therapy, further phenytoin medication is contraindicated. Phenytoin and other hydantoins are contraindicated in patients who have experienced phenytoin hypersensitivity (see CONTRAINDICATIONS). Additionally, caution should be exercised if using structurally similar (e.g., barbiturates, succinimides, oxazolidinediones, and other related compounds) in these same patients. Hyperglycemia, resulting from the drug's inhibitory effects on insulin release, has been reported. Phenytoin may also raise the serum glucose level in diabetic patients. Osteomalacia has been associated with phenytoin therapy and is considered to be due to phenytoin's interference with vitamin D metabolism. Phenytoin is not indicated for seizures due to hypoglycemic or other metabolic causes. Appropriate diagnostic procedures should be performed as indicated. Phenytoin is not effective for absence (petit mal) seizures. If tonic-clonic (grand mal) and absence (petit mal) seizures are present, combined drug therapy is needed. Serum levels of phenytoin sustained above the optimal range may produce confusional states referred to as "delirium," "psychosis," or "encephalopathy," or rarely irreversible cerebellar dysfunction. Accordingly, at the first sign of acute toxicity, plasma levels are recommended. Dose reduction of phenytoin therapy is indicated if plasma levels are excessive; if symptoms persist, termination is recommended. (See WARNINGS section.) Information for PatientsInform patients of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking Dilantin. Instruct patients to take Dilantin only as prescribed. Patients taking phenytoin should be advised of the importance of adhering strictly to the prescribed dosage regimen, and of informing the physician of any clinical condition in which it is not possible to take the drug orally as prescribed, e.g., surgery, etc. Patients should also be cautioned on the use of other drugs or alcoholic beverages without first seeking the physician's advice. Patients should be instructed to call their physician if skin rash develops. The importance of good dental hygiene should be stressed in order to minimize the development of gingival hyperplasia and its complications. Patients, their caregivers, and families should be counseled that AEDs, including Dilantin Infatabs, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers. Patients should be encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 (see PRECAUTIONS: Pregnancy section). Laboratory TestsPhenytoin serum level determinations may be necessary to achieve optimal dosage adjustments. Drug InteractionsThere are many drugs which may increase or decrease phenytoin levels or which phenytoin may affect. Serum level determinations for phenytoin are especially helpful when possible drug interactions are suspected. The most commonly occurring drug interactions are listed below:
Drug Enteral Feeding/Nutritional Preparations InteractionLiterature reports suggest that patients who have received enteral feeding preparations and/or related nutritional supplements have lower than expected phenytoin plasma levels. It is therefore suggested that phenytoin not be administered concomitantly with an enteral feeding preparation. More frequent serum phenytoin levels monitoring may be necessary in these patients. Drug/Laboratory Test InteractionsPhenytoin may decrease serum concentrations of T4. It may also produce lower than normal values for dexamethasone or metyrapone tests. Phenytoin may cause increased serum levels of glucose, alkaline phosphatase, and gamma glutamyl transpeptidase (GGT). Care should be taken when using immunoanalytical methods to measure plasma phenytoin concentrations. CarcinogenesisSee WARNINGS section for information on carcinogenesis. PregnancyPregnancy Category D: See WARNINGS section. To provide information regarding the effects of in utero exposure to Dilantin Infatabs, physicians are advised to recommend that pregnant patients taking Dilantin Infatabs enroll in the NAAED Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/. Nursing MothersInfant breast-feeding is not recommended for women taking this drug because phenytoin appears to be secreted in low concentrations in human milk. Pediatric UseSee DOSAGE AND ADMINISTRATION section. ADVERSE REACTIONSCentral Nervous SystemThe most common manifestations encountered with phenytoin therapy are referable to this system and are usually dose-related. These include nystagmus, ataxia, slurred speech, decreased coordination, and mental confusion. Dizziness, insomnia, transient nervousness, motor twitchings, and headache have also been observed. There have also been rare reports of phenytoin-induced dyskinesias, including chorea, dystonia, tremor and asterixis, similar to those induced by phenothiazine and other neuroleptic drugs. A predominantly sensory peripheral polyneuropathy has been observed in patients receiving long-term phenytoin therapy. Gastrointestinal SystemNausea, vomiting, constipation, toxic hepatitis and liver damage. Integumentary SystemDermatological manifestations sometimes accompanied by fever have included scarlatiniform or morbilliform rashes. A morbilliform rash (measles-like) is the most common; other types of dermatitis are seen more rarely. Other more serious forms which may be fatal have included bullous, exfoliative or purpuric dermatitis, lupus erythematosus, Stevens-Johnson syndrome, and toxic epidermal necrolysis (see PRECAUTIONS and WARNINGS section). Hemopoietic SystemHemopoietic complications, some fatal, have occasionally been reported in association with administration of phenytoin. These have included thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, and pancytopenia with or without bone marrow suppression. While macrocytosis and megaloblastic anemia have occurred, these conditions usually respond to folic acid therapy. Lymphadenopathy including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin's disease have been reported (see WARNINGS section). Connective Tissue SystemCoarsening of the facial features, enlargement of the lips, gingival hyperplasia, hypertrichosis, and Peyronie's disease. ImmunologicAnticonvulsant Hypersensitivity Syndrome (AHS) (which may include, but is not limited to, symptoms such as arthralgias, eosinophilia, fever, liver dysfunction, lymphadenopathy, or rash), systemic lupus erythematosus, periarteritis nodosa and immunoglobulin abnormalities (See WARNINGS section). OVERDOSAGEThe lethal dose in pediatric patients is not known. The lethal dose in adults is estimated to be 2 to 5 grams. The initial symptoms are nystagmus, ataxia, and dysarthria. Other signs are tremor, hyperreflexia, lethargy, slurred speech, nausea, vomiting. The patient may become comatose and hypotensive. Death is due to respiratory and circulatory depression. There are marked variations among individuals with respect to phenytoin plasma levels where toxicity may occur. Nystagmus on lateral gaze usually appears at 20 mcg/mL, ataxia at 30 mcg/mL, dysarthria and lethargy appear when the plasma concentration is over 40 mcg/mL, but as high a concentration as 50 mcg/mL has been reported without evidence of toxicity. As much as 25 times the therapeutic dose has been taken to result in a serum concentration over 100 mcg/mL with complete recovery. TreatmentTreatment is nonspecific since there is no known antidote. The adequacy of the respiratory and circulatory systems should be carefully observed and appropriate supportive measures employed. Hemodialysis can be considered since phenytoin is not completely bound to plasma proteins. Total exchange transfusion has been used in the treatment of severe intoxication in pediatric patients. In acute overdosage the possibility of other CNS depressants, including alcohol, should be borne in mind. DOSAGE AND ADMINISTRATIONWhen given in equal doses, Dilantin Infatabs yield higher plasma levels than Dilantin Kapseals®. For this reason serum concentrations should be monitored and care should be taken when switching a patient from the sodium salt to the free acid form. Dilantin® Kapseals® is formulated with the sodium salt of phenytoin. The free acid form of phenytoin is used in Dilantin-125 Suspensions and Dilantin Infatabs. Because there is approximately an 8% increase in drug content with the free acid form over that of the sodium salt, dosage adjustments and serum level monitoring may be necessary when switching from a product formulated with the free acid to a product formulated with the sodium salt and vice versa. GeneralNot for once-a-day dosing. Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations may be necessary for optimal dosage adjustments—the clinically effective serum level is usually 10–20 mcg/mL. With recommended dosage, a period of seven to ten days may be required to achieve steady-state blood levels with phenytoin and changes in dosage (increase or decrease) should not be carried out at intervals shorter than seven to ten days. Dilantin Infatabs can be either chewed thoroughly before being swallowed or swallowed whole. Adult DosagePatients who have received no previous treatment may be started on two Infatabs three times daily, and the dose is then adjusted to suit individual requirements. For most adults, the satisfactory maintenance dosage will be six to eight Infatabs daily; an increase to twelve Infatabs daily may be made, if necessary. Pediatric DosageInitially, 5 mg/kg/day in two or three equally divided doses, with subsequent dosage individualized to a maximum 300 mg daily. A recommended daily maintenance dosage is usually 4 to 8 mg/kg. Children over 6 years old and adolescents may require the minimum adult dose (300 mg/day). If the daily dosage cannot be divided equally, the larger dose should be given before retiring. HOW SUPPLIEDDilantin Infatabs are supplied as: N 0071-0007-24—Bottle of 100. Store at a room temperature below 30°C (86°F). N 0071-0007-40—Unit dose (10/10's). Store at controlled room temperature 15°–30°C (59°–86°F). Each tablet contains 50 mg phenytoin in a yellow triangular scored chewable tablet.
LAB-0204-6.0 April 2011 MEDICATION GUIDEDILANTIN (Dī lan' tĭn) Oral Suspension, Tablets, Extended Oral Capsules Read this Medication Guide before you start taking DILANTIN and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about DILANTIN, ask your healthcare provider or pharmacist. What is the most important information I should know about DILANTIN? Do not stop taking DILANTIN without first talking to your healthcare provider. Stopping DILANTIN suddenly can cause serious problems. DILANTIN can cause serious side effects including:
How can I watch for early symptoms of suicidal thoughts and actions?
Call your healthcare provider between visits as needed, especially if you are worried about symptoms. Do not stop taking DILANTIN without first talking to a healthcare provider.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
Call your healthcare provider right away if you have any of the symptoms listed above. What is DILANTIN? DILANTIN is a prescription medicine used to treat tonic-clonic (grand mal), complex partial (psychomotor or temporal lobe) seizures, and to prevent and treat seizures that happen during or after brain surgery. Who should not take DILANTIN? Do not take DILANTIN if you:
What should I tell my healthcare provider before taking DILANTIN? Before you take DILANTIN, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Taking DILANTIN with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine. How should I take DILANTIN?
What should I avoid while taking DILANTIN? Do not drink alcohol while you take DILANTIN without first talking to your healthcare provider. Drinking alcohol while taking DILANTIN may change your blood levels of DILANTIN which can cause serious problems. Do not drive, operate heavy machinery, or do other dangerous activities until you know how DILANTIN affects you. DILANTIN can slow your thinking and motor skills. What are the possible side effects of DILANTIN? See "What is the most important information I should know about DILANTIN?" DILANTIN may cause other serious side effects including:
Call your healthcare provider right away, if you have any of the symptoms listed above. The most common side effects of DILANTIN include:
These are not all the possible side effects of DILANTIN. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store DILANTIN?
Keep DILANTIN and all medicines out of the reach of children. General information about DILANTIN Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use DILANTIN for a condition for which it was not prescribed. Do not give DILANTIN to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about DILANTIN. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about DILANTIN that was written for healthcare professionals. For more information about DILANTIN, visit http://www.pfizer.com or call 1-800-438-1985. What are the ingredients in DILANTIN? Oral Suspension Active ingredient: phenytoin Inactive ingredients: USP; alcohol, USP (maximum content not greater than 0.6 percent); banana flavor; carboxymethylcellulose sodium, USP; citric acid, anhydrous, USP; glycerin, USP; magnesium aluminum silicate, NF; orange oil concentrate; polysorbate 40, NF; purified water, USP; sodium benzoate, NF; sucrose, NF; vanillin, NF; and FD&C yellow No. 6. Tablet Each tablet is a yellow triangular scored chewable tablet. Active ingredient: 50 mg phenytoin Inactive ingredients: D & C yellow No. 10, A1 lake, FD&C yellow No. 6, flavor, saccharin sodium, sucrose, talc, and other ingredients. Extended Oral Capsule Dilantin 100mg: Each capsule contains a white powder. The medium orange cap has "PD" imprinted in black ink and the white, opaque body has "DILANTIN" over "100 mg" printed in black ink. Active ingredient: 100 mg phenytoin sodium Inactive ingredients: lactose monohydrate, confectioner's sugar, talc, and magnesium stearate. The capsule body contains titanium dioxide and gelatin. The capsule cap contains FD&C red No. 28, FD&C yellow No. 6, and gelatin. Dilantin 30mg: Each capsule contains a white powder. The small pale pink opaque cap has "PD" imprinted in black ink and the white, opaque body has "Dilantin 30 mg" printed in black ink. Active ingredient: 30 mg phenytoin sodium Inactive ingredients: lactose monohydrate, confectioner's sugar, talc, and magnesium stearate. The capsule shell cap and body contain Titanium Dioxide (cap and body); gelatin (cap and body); D&C yellow No. 10 (cap); FD&C red No. 3 (cap). This Medication Guide has been approved by the U.S. Food and Drug Administration.
LAB-0398-1.0 January 2011 PRINCIPAL DISPLAY PANEL - 50 mg Tablet CartonNDC 0071-0007-40 100 Tablets INFATABS® 50 mg |
|
当前位置:药品说明书与价格首页 >> 神经内科 >> 癫痫 >> 药品推荐 >> 苯妥酸咀嚼片|Dilantin Infatabs (Phenytoin acid form Chewable Tablets)
苯妥酸咀嚼片|Dilantin Infatabs (Phenytoin acid form Chewable Tablets)简介:
英文药名: Dilantin Infatabs (Phenytoin acid form Chewable Tablets)
中文药名: 苯妥英钠(苯妥酸咀嚼片)
生产品牌药厂家: Pfizer
药品名称
别名: 大仑丁, 苯妥英钠,奇非宁, 大伦丁, 二苯乙内酰 ... 责任编辑:admin |
最新文章更多推荐文章更多热点文章更多 |



