|
新类型混合吸入粉剂ANORO ELLIPTA(umeclidinium and vilanterol inhalation powder) 获美国FDA批准用于治疗慢性阻塞性肺疾病
2013年12月22日,美国药品食品监督局(FDA)批准Anoro Ellipta(umeclidinium和vilanterol的混合吸入粉剂)作为慢性阻塞性肺疾病(COPD)患者有气流受限的人的日常长期维持治疗用药。
COPD是一种能造成呼吸困难并随着时间推移进行性加重的严重肺部疾病。症状包括胸部紧迫感、慢性咳嗽和痰多。吸烟是导致COPD的首要原因。据美国国家心脏、肺和血液研究所称,在美国COPD是第三大死因。
“AnoroEllipta通过帮助舒张呼吸道周围的肌肉组织来增加COPD患者的通气量” FDA新药评审研究中心新药审评第二室主任,公共卫生学硕士和医学博士CurtisRosebraugh说,“这种有效的长期维护的新药为美国数以百万计的COPD患者提供了额外的治疗选择”
AnoroEllipta是umeclidinium和vilanterol的混合制剂。umeclidinium是一种作用于大气道周围平滑肌并阻断肌紧张的吸入性抗胆碱药。而vilanterol是一种长效β2受体激动药(LABA),可以通过舒张气道平滑肌使更多的气体进出肺组织以增强呼吸。
AnoroEllipta的安全性和有效性已经在2,400名确诊为COPD患者的身上得到了证实。治疗组与安慰剂组相比肺功能提高了。
这种药物携带有LABAs增加哮喘相关疾病死亡风险的黑框警告。AnoroEllipta对于哮喘患者的安全性和有效性尚未被确定,而且AnoroEllipta尚未被证实可以用于哮喘病的治疗。Anoro Ellipta不能用于急性呼吸问题(急性支气管痉挛)的急救治疗。
FDA核准了AnoroEllipta及其包括使用说明书和服药潜在风险信息在内的患者用药指导。AnoroEllipta可能造成严重的副作用,包括呼吸道狭窄和阻塞(矛盾性支气管痉挛)、心血管作用、眼压增高(狭角性青光眼)、尿潴留加重。使用AnoroEllipta被报道的最常见的副作用有咽喉痛(咽炎)、鼻窦感染(鼻窦炎)、下呼吸道感染、便秘、腹泻、疼痛、肌肉痉挛、项痛、胸痛。

HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use the ANORO ELLIPTA inhaler safely and effectively. See full prescribing information for ANORO ELLIPTA.
ANORO ELLIPTA (umeclidinium and vilanterol inhalation powder)
FOR ORAL INHALATION USE
Initial U.S. Approval: 2013
WARNING: ASTHMA-RELATED DEATHSee full prescribing information for complete boxed warning.
• Long-acting beta2-adrenergic agonists (LABA), such as vilanterol, one of the active ingredients in ANORO ELLIPTA, increase the risk of asthma-related death. A placebo-controlled trial with another LABA (salmeterol) showed an increase in asthma-related deaths in subjects receiving salmeterol. This finding with salmeterol is considered a class effect of all LABA, including vilanterol. (5.1)
• The safety and efficacy of ANORO ELLIPTA in patients with asthma have not been established. ANORO ELLIPTA is not indicated for the treatment of asthma. (5.1)
INDICATIONS AND USAGE
ANORO ELLIPTA is a combination of umeclidinium, an anticholinergic, and vilanterol, a long-acting beta2-adrenergic agonist (LABA), indicated for the long-term, once-daily, maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD). (1)
Important limitations: Not indicated for the relief of acute bronchospasm or for the treatment of asthma. (1, 5.2)
DOSAGE AND ADMINISTRATION
• For oral inhalation only. ( 2)
• Maintenance treatment of COPD: 1 inhalation of ANORO ELLIPTA once daily. ( 2)
DOSAGE FORMS AND STRENGTHS
Inhalation Powder. Inhaler containing 2 double-foil blister strips of powder formulation for oral inhalation. One strip contains umeclidinium 62.5 mcg per blister and the other contains vilanterol 25 mcg per blister. (3)
CONTRAINDICATIONS
• Severe hypersensitivity to milk proteins or any ingredients. ( 4)
WARNINGS AND PRECAUTIONS
• LABA increase the risk of asthma-related death. ( 5.1)
• Do not initiate in acutely deteriorating COPD or to treat acute symptoms. ( 5.2)
• Do not use in combination with an additional medicine containing LABA because of risk of overdose. ( 5.3)
• If paradoxical bronchospasm occurs, discontinue ANORO ELLIPTA and institute alternative therapy. ( 5.5)
• Use with caution in patients with cardiovascular disorders ( 5.7)
• Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis. ( 5.8)
• Worsening of narrow-angle glaucoma may occur. Use with caution in patients with narrow-angle glaucoma and instruct patients to contact a physician immediately if symptoms occur. ( 5.9)
• Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to contact a physician immediately if symptoms occur. ( 5.10)
• Be alert to hypokalemia and hyperglycemia. ( 5.11)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥1% and more common than placebo) include pharyngitis, sinusitis, lower respiratory tract infection, constipation, diarrhea, pain in extremity, muscle spasms, neck pain, and chest pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch.
DRUG INTERACTIONS
• Strong cytochrome P450 3A4 inhibitors (e.g., ketoconazole): Use with caution. May cause cardiovascular effects. ( 7.1)
• Monoamine oxidase inhibitors and tricyclic antidepressants: Use with extreme caution. May potentiate effect of vilanterol on cardiovascular system. ( 7.2)
• Beta-blockers: Use with caution. May block bronchodilatory effects of beta-agonists and produce severe bronchospasm. ( 7.3)
• Diuretics: Use with caution. Electrocardiographic changes and/or hypokalemia associated with non–potassium-sparing diuretics may worsen with concomitant beta-agonists. ( 7.4)
• Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of ANORO ELLIPTA with other anticholinergic-containing drugs. ( 7.5)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2014
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
ANORO ELLIPTA is a combination anticholinergic/long-acting beta2-adrenergic agonist (anticholinergic/LABA) indicated for the long-term, once-daily, maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Important Limitations of Use: ANORO ELLIPTA is NOT indicated for the relief of acute bronchospasm or for the treatment of asthma.
2 DOSAGE AND ADMINISTRATION
ANORO ELLIPTA (umeclidinium/vilanterol 62.5 mcg/25 mcg) should be administered as 1 inhalation once daily by the orally inhaled route only.
ANORO ELLIPTA should be taken at the same time every day. Do not use ANORO ELLIPTA more than 1 time every 24 hours.
No dosage adjustment is required for geriatric patients, patients with renal impairment, or patients with moderate hepatic impairment [see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
Inhalation Powder. Disposable light grey and red plastic inhaler containing 2 double-foil blister strips, each with 30 blisters containing powder intended for oral inhalation only. One strip contains umeclidinium (62.5 mcg per blister), and the other strip contains vilanterol (25 mcg per blister). An institutional pack containing 7 blisters per strip is also available.
4 CONTRAINDICATIONS
The use of ANORO ELLIPTA is contraindicated in patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to umeclidinium, vilanterol, or any of the excipients [see Warnings and Precautions (5.6), Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Asthma-Related Death
• Data from a large placebo-controlled trial in subjects with asthma showed that LABA may increase the risk of asthma-related death. Data are not available to determine whether the rate of death in patients with COPD is increased by LABA.
• A 28-week, placebo-controlled, US trial comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in subjects receiving salmeterol (13/13,176 in subjects treated with salmeterol vs. 3/13,179 in subjects treated with placebo; relative risk: 4.37 [95% CI: 1.25, 15.34]). The increased risk of asthma-related death is considered a class effect of LABA, including vilanterol, one of the active ingredients in ANORO ELLIPTA.
• No trial adequate to determine whether the rate of asthma-related death is increased in subjects treated with ANORO ELLIPTA has been conducted. The safety and efficacy of ANORO ELLIPTA in patients with asthma have not been established. ANORO ELLIPTA is not indicated for the treatment of asthma.
5.2 Deterioration of Disease and Acute Episodes
ANORO ELLIPTA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD. ANORO ELLIPTA has not been studied in subjects with acutely deteriorating COPD. The initiation of ANORO ELLIPTA in this setting is not appropriate.
ANORO ELLIPTA should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. ANORO ELLIPTA has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
When beginning treatment with ANORO ELLIPTA, patients who have been taking oral or inhaled, short-acting beta2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs and to use them only for symptomatic relief of acute respiratory symptoms. When prescribing ANORO ELLIPTA, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist and instruct the patient on how it should be used. Increasing inhaled, short-acting beta2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If ANORO ELLIPTA no longer controls symptoms of bronchoconstriction; the patient’s inhaled, short-acting beta2-agonist becomes less effective; or the patient needs more short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dose of ANORO ELLIPTA beyond the recommended dose is not appropriate in this situation.
5.3 Excessive Use of ANORO ELLIPTA and Use With Other Long-Acting Beta2-Agonists
ANORO ELLIPTA should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medicines containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using ANORO ELLIPTA should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
5.4 Drug Interactions With Strong Cytochrome P450 3A4 Inhibitors
Caution should be exercised when considering the coadministration of ANORO ELLIPTA with long-term ketoconazole and other known strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, clarithromycin, conivaptan, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, saquinavir, telithromycin, troleandomycin, voriconazole) because increased cardiovascular adverse effects may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
5.5 Paradoxical Bronchospasm
As with other inhaled medicines, ANORO ELLIPTA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with ANORO ELLIPTA, it should be treated immediately with an inhaled, short-acting bronchodilator; ANORO ELLIPTA should be discontinued immediately; and alternative therapy should be instituted.
5.6 Hypersensitivity Reactions
Hypersensitivity reactions may occur after administration of ANORO ELLIPTA. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder products containing lactose; therefore, patients with severe milk protein allergy should not use ANORO ELLIPTA [see Contraindications (4)].
5.7 Cardiovascular Effects
Vilanterol, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, or symptoms [see Clinical Pharmacology (12.2)]. If such effects occur, ANORO ELLIPTA may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiographic changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown.
Therefore, ANORO ELLIPTA should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
5.8 Coexisting Conditions
ANORO ELLIPTA, like all medicines containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2-adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.9 Worsening of Narrow-Angle Glaucoma
ANORO ELLIPTA should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
5.10 Worsening of Urinary Retention
ANORO ELLIPTA should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
5.11 Hypokalemia and Hyperglycemia
Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. Beta-agonist medicines may produce transient hyperglycemia in some patients. In 4 clinical trials of 6-month duration evaluating ANORO ELLIPTA in subjects with COPD, there was no evidence of a treatment effect on serum glucose or potassium.
6 ADVERSE REACTIONS
LABA, such as vilanterol, one of the active ingredients in ANORO ELLIPTA, increase the risk of asthma-related death. ANORO ELLIPTA is not indicated for the treatment of asthma. [See Boxed Warning and Warnings and Precautions (5.1).]
The following adverse reactions are described in greater detail in other sections:
• Paradoxical bronchospasm [see Warnings and Precautions (5.5)]
• Cardiovascular effects [see Warnings and Precautions (5.7)]
• Worsening of narrow-angle glaucoma [see Warnings and Precautions (5.9)]
• Worsening of urinary retention [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical program for ANORO ELLIPTA included 8,138 subjects with COPD in four 6-month lung function trials, one 12-month long-term safety study, and 9 other trials of shorter duration. A total of 1,124 subjects have received at least 1 dose of ANORO ELLIPTA (umeclidinium/vilanterol 62.5 mcg/25 mcg), and 1,330 subjects have received a higher dose of umeclidinium/vilanterol (125 mcg/25 mcg). The safety data described below are based on the four 6-month and the one 12-month trials. Adverse reactions observed in the other trials were similar to those observed in the confirmatory trials.
6-Month Trials: The incidence of adverse reactions associated with ANORO ELLIPTA in Table 1 is based on four 6-month trials: 2 placebo-controlled trials (Trials 1 and 2; n = 1,532 and n = 1,489, respectively) and 2 active-controlled trials (Trials 3 and 4; n = 843 and n = 869, respectively). Of the 4,733 subjects, 68% were male and 84% were Caucasian. They had a mean age of 63 years and an average smoking history of 45 pack-years, with 50% identified as current smokers. At screening, the mean post-bronchodilator percent predicted forced expiratory volume in 1 second (FEV1) was 48% (range: 13% to 76%), the mean post-bronchodilator FEV1/forced vital capacity (FVC) ratio was 0.47 (range: 0.13 to 0.78), and the mean percent reversibility was 14% (range: -45% to 109%).
Subjects received 1 dose once daily of the following: ANORO ELLIPTA, umeclidinium/vilanterol 125 mcg/25 mcg, umeclidinium 62.5 mcg, umeclidinium 125 mcg, vilanterol 25 mcg, active control, or placebo.
Table 1. Adverse Reactions With ANORO ELLIPTA With ≥1% Incidence and More Common Than With Placebo in Subjects With Chronic Obstructive Pulmonary Disease
|
Adverse Reaction |
Placebo
(n = 555)
% |
ANORO ELLIPTA
(n = 842)
% |
Umeclidinium
62.5 mcg
(n = 418)
% |
Vilanterol
25 mcg
(n = 1,034)
% |
|
Infections and infestations |
|
|
|
|
- Pharyngitis
|
<1 |
2 |
1 |
2 |
- Sinusitis
|
<1 |
1 |
<1 |
1 |
- Lower respiratory tract infection
|
<1 |
1 |
<1 |
<1 |
|
Gastrointestinal disorders |
|
|
|
|
- Constipation
|
<1 |
1 |
<1 |
<1 |
- Diarrhea
|
1 |
2 |
<1 |
2 |
|
Musculoskeletal and connective tissue disorders |
|
|
|
|
- Pain in extremity
|
1 |
2 |
<1 |
2 |
- Muscle spasms
|
<1 |
1 |
<1 |
<1 |
- Neck pain
|
<1 |
1 |
<1 |
<1 |
|
General disorders and administration site conditions |
|
|
|
|
- Chest pain
|
<1 |
1 |
<1 |
<1 | Other adverse reactions with ANORO ELLIPTA observed with an incidence less than 1% but more common than with placebo included the following: productive cough, dry mouth, dyspepsia, abdominal pain, gastroesophageal reflux disease, vomiting, musculoskeletal chest pain, chest discomfort, asthenia, atrial fibrillation, ventricular extrasystoles, supraventricular extrasystoles, myocardial infarction, pruritus, rash, and conjunctivitis.
12-Month Trial: In a long-term safety trial, 335 subjects were treated for up to 12 months with umeclidinium/vilanterol 125 mcg/25 mcg or placebo. The demographic and baseline characteristics of the long-term safety trial were similar to those of the placebo-controlled efficacy trials described above. Adverse reactions that occurred with a frequency of greater than or equal to 1% in the group receiving umeclidinium/vilanterol 125 mcg/25 mcg that exceeded that in placebo in this trial were: headache, back pain, sinusitis, cough, urinary tract infection, arthralgia, nausea, vertigo, abdominal pain, pleuritic pain, viral respiratory tract infection, toothache, and diabetes mellitus.
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
Vilanterol, a component of ANORO ELLIPTA, is a substrate of CYP3A4. Concomitant administration of the strong CYP3A4 inhibitor ketoconazole increases the systemic exposure to vilanterol. Caution should be exercised when considering the coadministration of ANORO ELLIPTA with ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, clarithromycin, conivaptan, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, saquinavir, telithromycin, troleandomycin, voriconazole) [see Warnings and Precautions (5.4), Clinical Pharmacology (12.3)].
7.2 Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
Vilanterol, like other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval or within 2 weeks of discontinuation of such agents, because the effect of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval have an increased risk of ventricular arrhythmias.
7.3 Beta-Adrenergic Receptor Blocking Agents
Beta-blockers not only block the pulmonary effect of beta-agonists, such as vilanterol, a component of ANORO ELLIPTA, but may produce severe bronchospasm in patients with COPD. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents for these patients; cardioselective beta-blockers could be considered, although they should be administered with caution.
7.4 Non–Potassium-Sparing Diuretics
The electrocardiographic changes and/or hypokalemia that may result from the administration of non–potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, such as vilanterol, a component of ANORO ELLIPTA, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of ANORO ELLIPTA with non–potassium-sparing diuretics.
7.5 Anticholinergics
There is potential for an additive interaction with concomitantly used anticholinergic medicines. Therefore, avoid coadministration of ANORO ELLIPTA with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see Warnings and Precautions (5.9, 5.10), Adverse Reactions (6)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C. There are no adequate and well-controlled trials of ANORO ELLIPTA or its individual components, umeclidinium and vilanterol, in pregnant women. Because animal reproduction studies are not always predictive of human response, ANORO ELLIPTA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women should be advised to contact their physicians if they become pregnant while taking ANORO ELLIPTA.
Umeclidinium: There was no evidence of teratogenic effects in rats and rabbits at approximately 50 and 200 times, respectively, the MRHDID (maximum recommended human daily inhaled dose) in adults (on an AUC basis at maternal inhaled doses up to 278 mcg/kg/day in rats and at maternal subcutaneous doses up to 180 mcg/kg/day in rabbits).
Vilanterol: There were no teratogenic effects in rats and rabbits at approximately 13,000 and 70 times, respectively, the MRHDID in adults (on a mcg/m2 basis at maternal inhaled doses up to 33,700 mcg/kg/day in rats and on an AUC basis at maternal inhaled doses up to 591 mcg/kg/day in rabbits). However, fetal skeletal variations were observed in rabbits at approximately 450 times the MRHDID in adults (on an AUC basis at maternal inhaled or subcutaneous doses of 5,740 or 300 mcg/kg/day, respectively). The skeletal variations included decreased or absent ossification in cervical vertebral centrum and metacarpals.
Nonteratogenic Effects: Umeclidinium: There were no effects on perinatal and postnatal developments in rats at approximately 80 times the MRHDID in adults (on an AUC basis at maternal subcutaneous doses up to 180 mcg/kg/day).
Vilanterol: There were no effects on perinatal and postnatal developments in rats at approximately 3,900 times the MRHDID in adults (on a mcg/m2 basis at maternal oral doses up to 10,000 mcg/kg/day).
8.2 Labor and Delivery
There are no adequate and well-controlled human trials that have investigated the effects of ANORO ELLIPTA during labor and delivery.
Because beta-agonists may potentially interfere with uterine contractility, ANORO ELLIPTA should be used during labor only if the potential benefit justifies the potential risk.
8.3 Nursing Mothers
ANORO ELLIPTA: It is not known whether ANORO ELLIPTA is excreted in human breast milk. Because many drugs are excreted in human milk, caution should be exercised when ANORO ELLIPTA is administered to a nursing woman. Since there are no data from well-controlled human studies on the use of ANORO ELLIPTA by nursing mothers, based on the data for the individual components, a decision should be made whether to discontinue nursing or to discontinue ANORO ELLIPTA, taking into account the importance of ANORO ELLIPTA to the mother.
Umeclidinium: It is not known whether umeclidinium is excreted in human breast milk. However, administration to lactating rats at approximately 25 times the MRHDID in adults resulted in a quantifiable level of umeclidinium in 2 pups, which may indicate transfer of umeclidinium in milk.
Vilanterol: It is not known whether vilanterol is excreted in human breast milk. However, other beta2-agonists have been detected in human milk.
8.4 Pediatric Use
ANORO ELLIPTA is not indicated for use in children. The safety and efficacy in pediatric patients have not been established.
8.5 Geriatric Use
Based on available data, no adjustment of the dosage of ANORO ELLIPTA in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
Clinical trials of ANORO ELLIPTA for COPD included 2,143 subjects aged 65 and older and, of those, 478 subjects were aged 75 and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects.
8.6 Hepatic Impairment
Patients with moderate hepatic impairment (Child-Pugh score of 7-9) showed no relevant increases in Cmax or AUC, nor did protein binding differ between subjects with moderate hepatic impairment and their healthy controls. Studies in subjects with severe hepatic impairment have not been performed [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
There were no significant increases in either umeclidinium or vilanterol exposure in subjects with severe renal impairment (CrCl<30 mL/min) compared with healthy subjects. No dosage adjustment is required in patients with renal impairment [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
No case of overdose has been reported with ANORO ELLIPTA.
ANORO ELLIPTA contains both umeclidinium and vilanterol; therefore, the risks associated with overdosage for the individual components described below apply to ANORO ELLIPTA. Treatment of overdosage consists of discontinuation of ANORO ELLIPTA together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medicine can produce bronchospasm. Cardiac monitoring is recommended in cases of overdosage.
10.1 Umeclidinium
High doses of umeclidinium may lead to anticholinergic signs and symptoms. However, there were no systemic anticholinergic adverse effects following a once-daily inhaled dose of up to 1,000 mcg umeclidinium (16 times the maximum recommended daily dose) for 14 days in subjects with COPD.
10.2 Vilanterol
The expected signs and symptoms with overdosage of vilanterol are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms of beta-adrenergic stimulation (e.g., angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, seizures, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, hypokalemia, metabolic acidosis). As with all inhaled sympathomimetic medicines, cardiac arrest and even death may be associated with an overdose of vilanterol.
11 DESCRIPTION
ANORO ELLIPTA is an inhalation powder drug product for delivery of a combination of umeclidinium (an anticholinergic) and vilanterol (a LABA) to patients by oral inhalation.
Umeclidinium bromide has the chemical name 1-[2-(benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-azoniabicyclo[2.2.2]octane bromide and the following chemical structure:

Umeclidinium bromide is a white powder with a molecular weight of 508.5, and the empirical formula is C29H34NO2•Br (as a quaternary ammonium bromide compound). It is slightly soluble in water.
Vilanterol trifenatate has the chemical name triphenylacetic acid-4-{(1R)-2-[(6-{2-[(2,6-dicholorobenzyl)oxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol (1:1) and the following chemical structure:

Vilanterol trifenatate is a white powder with a molecular weight of 774.8, and the empirical formula is C24H33Cl2NO5•C20H16O2. It is practically insoluble in water.
ANORO ELLIPTA is a light grey and red plastic inhaler containing 2 double-foil blister strips. Each blister on one strip contains a white powder mix of micronized umeclidinium bromide (74.2 mcg equivalent to 62.5 mcg of umeclidinium), magnesium stearate (75 mcg), and lactose monohydrate (to 12.5 mg), and each blister on the other strip contains a white powder mix of micronized vilanterol trifenatate (40 mcg equivalent to 25 mcg of vilanterol), magnesium stearate (125 mcg), and lactose monohydrate (to 12.5 mg). The lactose monohydrate contains milk proteins. After the inhaler is activated, the powder within both blisters is exposed and ready for dispersion into the airstream created by the patient inhaling through the mouthpiece.
Under standardized in vitro test conditions, ANORO ELLIPTA delivers 55 mcg of umeclidinium and 22 mcg of vilanterol per dose when tested at a flow rate of 60 L/min for 4 seconds.
In adult subjects with obstructive lung disease and severely compromised lung function (COPD with FEV1/FVC less than 70% and FEV1 less than 30% predicted or FEV1 less than 50% predicted plus chronic respiratory failure), mean peak inspiratory flow through the ELLIPTA inhaler was 66.5 L/min (range: 43.5 to 81.0 L/min).
The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ANORO ELLIPTA: ANORO ELLIPTA contains both umeclidinium and vilanterol. The mechanisms of action described below for the individual components apply to ANORO ELLIPTA. These drugs represent 2 different classes of medications (an anticholinergic and a LABA) that have different effects on clinical and physiological indices.
Umeclidinium: Umeclidinium is a long-acting antimuscarinic agent, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of umeclidinium is predominantly a site-specific effect.
Vilanterol: Vilanterol is a LABA. In vitro tests have shown the functional selectivity of vilanterol was similar to salmeterol. The clinical relevance of this in vitro finding is unknown.
Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenergic agonist drugs, including vilanterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3’,5’-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
12.2 Pharmacodynamics
Cardiovascular Effects: Healthy Subjects: QTc interval prolongation was studied in a double-blind, multiple-dose, placebo- and positive-controlled crossover trial in 86 healthy subjects. The maximum mean (95% upper confidence bound) difference in QTcF from placebo after baseline correction was 4.6 (7.1) ms and 8.2 (10.7) ms for umeclidinium/vilanterol 125 mcg/25 mcg and umeclidinium/vilanterol 500 mcg/100 mcg (8/4 times the recommended dosage), respectively.
A dose-dependent increase in heart rate was also observed. The maximum mean (95% upper confidence bound) difference in heart rate from placebo after baseline correction was 8.8 (10.5) beats/min and 20.5 (22.3) beats/min seen 10 minutes after dosing for umeclidinium/vilanterol 125 mcg/25 mcg and umeclidinium/vilanterol 500 mcg/100 mcg, respectively.
Chronic Obstructive Pulmonary Disease: The effect of ANORO ELLIPTA on cardiac rhythm in subjects diagnosed with COPD was assessed using 24-hour Holter monitoring in 6- and 12-month trials: 53 subjects received ANORO ELLIPTA, 281 subjects received umeclidinium/vilanterol 125 mcg/25 mcg, and 182 subjects received placebo. No clinically meaningful effects on cardiac rhythm were observed.
12.3 Pharmacokinetics
Linear pharmacokinetics was observed for umeclidinium (62.5 to 500 mcg) and vilanterol (25 to 100 mcg).
Absorption: Umeclidinium: Umeclidinium plasma levels may not predict therapeutic effect. Following inhaled administration of umeclidinium in healthy subjects, Cmax occurred at 5 to 15 minutes. Umeclidinium is mostly absorbed from the lung after inhaled doses with minimum contribution from oral absorption. Following repeat dosing of inhaled ANORO ELLIPTA, steady state was achieved within 14 days with up to 1.8-fold accumulation.
Vilanterol: Vilanterol plasma levels may not predict therapeutic effect. Following inhaled administration of vilanterol in healthy subjects, Cmax occurred at 5 to 15 minutes. Vilanterol is mostly absorbed from the lung after inhaled doses with negligible contribution from oral absorption. Following repeat dosing of inhaled ANORO ELLIPTA, steady state was achieved within 14 days with up to 1.7-fold accumulation.
Distribution: Umeclidinium: Following intravenous administration to healthy subjects, the mean volume of distribution was 86 L. In vitro plasma protein binding in human plasma was on average 89%.
Vilanterol: Following intravenous administration to healthy subjects, the mean volume of distribution at steady state was 165 L. In vitro plasma protein binding in human plasma was on average 94%.
Metabolism: Umeclidinium: In vitro data showed that umeclidinium is primarily metabolized by the enzyme cytochrome P450 2D6 (CYP2D6) and is a substrate for the P-glycoprotein (P-gp) transporter. The primary metabolic routes for umeclidinium are oxidative (hydroxylation, O-dealkylation) followed by conjugation (e.g., glucuronidation), resulting in a range of metabolites with either reduced pharmacological activity or for which the pharmacological activity has not been established. Systemic exposure to the metabolites is low.
Vilanterol: In vitro data showed that vilanterol is metabolized principally by CYP3A4 and is a substrate for the P-gp transporter. Vilanterol is metabolized to a range of metabolites with significantly reduced β1- and β2-agonist activity.
Elimination: Umeclidinium: Following intravenous dosing with radio-labeled umeclidinium, mass balance showed 58% of the radio-label in the feces and 22% in the urine. The excretion of the drug-related material in the feces following intravenous dosing indicated elimination in the bile. Following oral dosing to healthy male subjects, radio-label recovered in feces was 92% of the total dose and that in urine was less than 1% of the total dose, suggesting negligible oral absorption. The effective half-life after once-daily dosing is 11 hours.
Vilanterol: Following oral administration of radio-labeled vilanterol, mass balance showed 70% of the radio-label in the urine and 30% in the feces. The effective half-life for vilanterol, as determined from inhalation administration of multiple doses, is 11 hours.
Special Populations: The effects of renal and hepatic impairment and other intrinsic factors on the pharmacokinetics of umeclidinium and vilanterol are shown in Figure 1. Population pharmacokinetic analysis showed no evidence of a clinically significant effect of age (40 to 93 years) (see Figure 1), gender (69% male) (see Figure 1), inhaled corticosteroid use (48%), or weight (34 to 161 kg) on systemic exposure of either umeclidinium or vilanterol. In addition, there was no evidence of a clinically significant effect of race.
Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Umeclidinium and Vilanterol

Hepatic Impairment: The impact of hepatic impairment on the pharmacokinetics of ANORO ELLIPTA has been evaluated in subjects with moderate hepatic impairment (Child-Pugh score of 7-9). There was no evidence of an increase in systemic exposure to either umeclidinium or vilanterol (Cmax and AUC) (see Figure 1). There was no evidence of altered protein binding in subjects with moderate hepatic impairment compared with healthy subjects. ANORO ELLIPTA has not been evaluated in subjects with severe hepatic impairment.
Renal Impairment: The pharmacokinetics of ANORO ELLIPTA has been evaluated in subjects with severe renal impairment (creatinine clearance <30 mL/min). Umeclidinium systemic exposure was not increased and vilanterol systemic exposure (AUC(0-24)) was 56% higher in subjects with severe renal impairment compared with healthy subjects (see Figure 1). There was no evidence of altered protein binding in subjects with severe renal impairment compared with healthy subjects.
Drug Interactions: When umeclidinium and vilanterol were administered in combination by the inhaled route, the pharmacokinetic parameters for each component were similar to those observed when each active substance was administered separately.
Inhibitors of Cytochrome P450 3A4: Vilanterol is a substrate of CYP3A4. A double-blind, repeat-dose, 2-way crossover drug interaction trial was conducted in healthy subjects to investigate the pharmacokinetic and pharmacodynamic effects of vilanterol 25 mcg as an inhalation powder with ketoconazole 400 mg. The plasma concentrations of vilanterol were higher after single and repeated doses when coadministered with ketoconazole than with placebo (see Figure 2). The increase in vilanterol exposure was not associated with an increase in beta-agonist–related systemic effects on heart rate or blood potassium.
Inhibitors of P-glycoprotein Transporter: Umeclidinium and vilanterol are both substrates of P-gp. The effect of the moderate P-gp transporter inhibitor verapamil (240 mg once daily) on the steady-state pharmacokinetics of umeclidinium and vilanterol was assessed in healthy subjects. No effect on umeclidinium or vilanterol Cmax was observed; however, an approximately 1.4-fold increase in umeclidinium AUC was observed with no effect on vilanterol AUC (see Figure 2).
Inhibitors of Cytochrome P450 2D6: In vitro metabolism of umeclidinium is mediated primarily by CYP2D6. However, no clinically meaningful difference in systemic exposure to umeclidinium (500 mcg) (8 times the approved dose) was observed following repeat daily inhaled dosing in CYP2D6 normal (ultrarapid, extensive, and intermediate metabolizers) and poor metabolizer subjects (see Figure 1).
Figure 2. Impact of Extrinsic Factors on the Pharmacokinetics (PK) of Umeclidinium and Vilanterol

13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
ANORO ELLIPTA: No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with ANORO ELLIPTA; however, studies are available for individual components, umeclidinium and vilanterol, as described below.
Umeclidinium: Umeclidinium produced no treatment-related increases in the incidence of tumors in 2-year inhalation studies in rats and mice at inhaled doses up to 137 mcg/kg/day and 295/200 mcg/kg/day (male/female), respectively (approximately 20 and 25/20 times the MRHDID in adults on an AUC basis, respectively).
Umeclidinium tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro mouse lymphoma assay, and in vivo rat bone marrow micronucleus assay.
No evidence of impairment of fertility was observed in male and female rats at subcutaneous doses up to 180 mcg/kg/day and inhaled doses up to 294 mcg/kg/day, respectively (approximately 100 and 50 times, respectively, the MRHDID in adults on an AUC basis).
Vilanterol: In a 2-year carcinogenicity study in mice, vilanterol caused a statistically significant increase in ovarian tubulostromal adenomas in females at an inhalation dose of 29,500 mcg/kg/day (approximately 7,800 times the MRHDID in adults on an AUC basis). No increase in tumors was seen at an inhalation dose of 615 mcg/kg/day (approximately 210 times the MRHDID in adults on an AUC basis).
In a 2-year carcinogenicity study in rats, vilanterol caused statistically significant increases in mesovarian leiomyomas in females and shortening of the latency of pituitary tumors at inhalation doses greater than or equal to 84.4 mcg/kg/day (greater than or equal to approximately 20 times the MRHDID in adults on an AUC basis). No tumors were seen at an inhalation dose of 10.5 mcg/kg/day (approximately 1 time the MRHDID in adults on an AUC basis).
These tumor findings in rodents are similar to those reported previously for other beta-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Vilanterol tested negative in the following genotoxicity assays: the in vitro Ames assay, in vivo rat bone marrow micronucleus assay, in vivo rat unscheduled DNA synthesis (UDS) assay, and in vitro Syrian hamster embryo (SHE) cell assay. Vilanterol tested equivocal in the in vitro mouse lymphoma assay.
No evidence of impairment of fertility was observed in reproductive studies conducted in male and female rats at inhaled vilanterol doses up to 31,500 and 37,100 mcg/kg/day, respectively (approximately 12,000 and 14,500 times, respectively, the MRHDID in adults on a mcg/m2 basis).
14 CLINICAL STUDIES
The safety and efficacy of ANORO ELLIPTA were evaluated in a clinical development program that included 6 dose-ranging trials, 4 lung function trials of 6 months’ duration (2 placebo-controlled and 2 active-controlled), two 12-week crossover trials, and a 12-month long-term safety trial. The efficacy of ANORO ELLIPTA is based primarily on the dose-ranging trials in 1,908 subjects with COPD or asthma and the 2 placebo-controlled confirmatory trials with additional support from the 2 active-controlled and 2 crossover trials in 5,388 subjects with COPD.
14.1 Dose-Ranging Trials
Dose selection for ANORO ELLIPTA for COPD was based on dose-ranging trials for the individual components, vilanterol and umeclidinium. Based on the findings from these studies, once-daily doses of umeclidinium/vilanterol 62.5 mcg/25 mcg and umeclidinium/vilanterol 125 mcg/25 mcg were evaluated in the confirmatory COPD trials. ANORO ELLIPTA is not indicated for asthma.
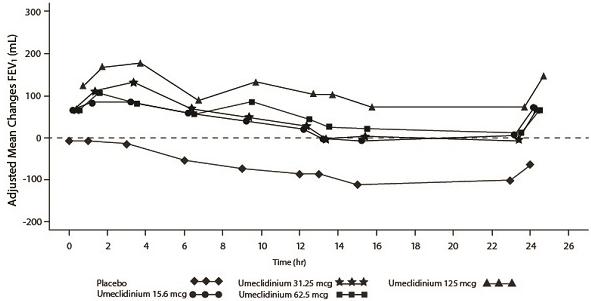
Umeclidinium: Dose selection for umeclidinium in COPD was supported by a 7-day, randomized, double-blind, placebo-controlled, crossover trial evaluating 4 doses of umeclidinium (15.6 to 125 mcg) or placebo dosed once daily in the morning in 163 subjects with COPD. A dose ordering was observed, with the 62.5- and 125-mcg doses demonstrating larger improvements in FEV1 over 24 hours compared with the lower doses of 15.6 and 31.25 mcg (Figure 3).
The differences in trough FEV1 from baseline after 7 days for placebo and the 15.6-, 31.25-, 62.5-, and 125-mcg doses were -74 mL (95% CI: -118, -31), 38 mL (95% CI: -6, 83), 27 mL (95% CI: -18, 72), 49 mL (95% CI: 6, 93), and 109 mL (95% CI: 65, 152), respectively. Two additional dose-ranging trials in subjects with COPD demonstrated minimal additional benefit at doses above 125 mcg. The dose-ranging results supported the evaluation of 2 doses of umeclidinium, 62.5 and 125 mcg, in the confirmatory COPD trials to further assess dose response.
Evaluations of dosing interval by comparing once- and twice-daily dosing supported selection of a once-daily dosing interval for further evaluation in the confirmatory COPD trials.
Figure 3. Adjusted Mean Change From Baseline in Post-Dose Serial FEV1 (mL) on Days 1 and 7

Day 7
Vilanterol: Dose selection for vilanterol in COPD was supported by a 28-day, randomized, double-blind, placebo-controlled, parallel-group trial evaluating 5 doses of vilanterol (3 to 50 mcg) or placebo dosed in the morning in 602 subjects with COPD. Results demonstrated dose-related increases in FEV1 compared with placebo at Day 1 and Day 28 (Figure 4).
Figure 4. Adjusted Mean Change From Baseline in Post-Dose Serial FEV1 (0-24 hr, mL) on Days 1 and 28
Day 1

Day 28
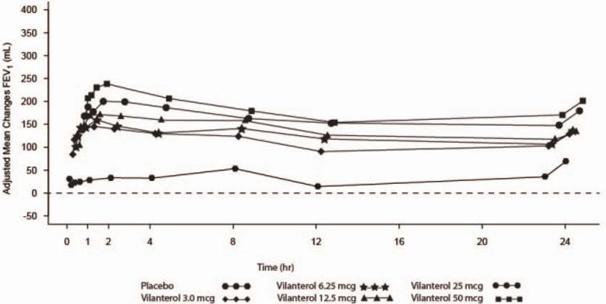
The differences in trough FEV1 after Day 28 from baseline for placebo and the 3-, 6.25-, 12.5-, 25-, and 50-mcg doses were 29 mL (95% CI: -8, 66), 120 mL (95% CI: 83, 158), 127 mL (95% CI: 90, 164), 138 mL (95% CI: 101, 176), 166 mL (95% CI: 129, 203), and 194 mL (95% CI: 156, 231), respectively. These results supported the evaluation of vilanterol 25 mcg in the confirmatory COPD trials.
Dose-ranging trials in subjects with asthma evaluated doses from 3 to 50 mcg and 12.5 mcg once-daily versus 6.25 mcg twice-daily dosing frequency. The results supported the selection of the vilanterol 25 mcg once-daily dose for further evaluation in the confirmatory COPD trials.
14.2 Confirmatory Trials
The clinical development program for ANORO ELLIPTA included two 6-month, randomized, double-blind, placebo-controlled, parallel-group trials; two 6-month active-controlled trials; and two 12-week crossover trials in subjects with COPD designed to evaluate the efficacy of ANORO ELLIPTA on lung function. The 6-month trials treated 4,733 subjects that had a clinical diagnosis of COPD, were 40 years of age or older, had a history of smoking greater than or equal to 10 pack-years, had a post-albuterol FEV1 less than or equal to 70% of predicted normal values, had a ratio of FEV1/FVC of less than 0.7, and had a Modified Medical Research Council (mMRC) score greater than or equal to 2. Of the 4,713 subjects included in the efficacy analysis, 68% were male and 84% were Caucasian. They had a mean age of 63 years and an average smoking history of 45 pack-years, with 50% identified as current smokers. At screening, the mean post-bronchodilator percent predicted FEV1 was 48% (range: 13% to 76%), the mean post-bronchodilator FEV1/FVC ratio was 0.47 (range: 0.13 to 0.78), and the mean percent reversibility was 14% (range: -36% to 109%).
Trial 1 evaluated ANORO ELLIPTA (umeclidinium/vilanterol 62.5 mcg/25 mcg), umeclidinium 62.5 mcg, vilanterol 25 mcg, and placebo. The primary endpoint was change from baseline in trough (predose) FEV1 at Day 169 (defined as the mean of the FEV1 values obtained at 23 and 24 hours after the previous dose on Day 168) compared with placebo, umeclidinium 62.5 mcg, and vilanterol 25 mcg. The comparison of ANORO ELLIPTA with umeclidinium 62.5 mcg and vilanterol 25 mcg was assessed to evaluate the contribution of the individual comparators to ANORO ELLIPTA. ANORO ELLIPTA demonstrated a larger increase in mean change from baseline in trough (predose) FEV1 relative to placebo, umeclidinium 62.5 mcg, and vilanterol 25 mcg (Table 2).
Table 2. Least Squares (LS) Mean Change From Baseline in Trough FEV1 (mL) at Day 169 in the Intent-to-Treat Population (Trial 1)
|
Treatment |
n |
Trough FEV1 (mL) at Day 169 |
|
Difference From |
|
Placebo
(95% CI)
n = 280 |
Umeclidinium
62.5 mcga
(95% CI)
n = 418 |
Vilanterol
25 mcga
(95% CI)
n = 421 |
|
ANORO ELLIPTA |
413 |
167
(128, 207) |
52
(17, 87) |
95
(60, 130) | n = Number in intent-to-treat population.
a The umeclidinium and vilanterol comparators used the same inhaler and excipients as ANORO ELLIPTA.
Trial 2 had a similar study design as Trial 1 but evaluated umeclidinium/vilanterol 125 mcg/25 mcg, umeclidinium 125 mcg, vilanterol 25 mcg, and placebo. Results for umeclidinium/vilanterol 125 mcg/25 mcg in Trial 2 were similar to those observed for ANORO ELLIPTA in Trial 1.
Results from the two active-controlled trials and the two 12-week trials provided additional support for the efficacy of ANORO ELLIPTA in terms of change from baseline in trough FEV1 compared with the single-ingredient comparators and placebo.
Serial spirometric evaluations throughout the 24-hour dosing interval were performed in a subset of subjects (n = 197) at Days 1, 84, and 168 in Trial 1. Results from Trial 1 at Day 1 and Day 168 are shown in Figure 5.
Figure 5. Least Squares (LS) Mean Change From Baseline in FEV1 (mL) Over Time (0-24 h) on Days 1 and 168 (Trial 1 Subset Population)
Day 1

Day 168

The peak FEV1 was defined as the maximum FEV1 recorded within 6 hours after the dose of trial medicine on Days 1, 28, 84, and 168 (measurements recorded at 15 and 30 minutes and 1, 3, and 6 hours). The mean peak FEV1 improvement from baseline for ANORO ELLIPTA compared with placebo at Day 1 and at Day 168 was 167 and 224 mL, respectively. The median time to onset on Day 1, defined as a 100-mL increase from baseline in FEV1, was 27 minutes in subjects receiving ANORO ELLIPTA.
16 HOW SUPPLIED/STORAGE AND HANDLING
ANORO ELLIPTA is supplied as a disposable light grey and red plastic inhaler containing 2 double-foil blister strips with 30 blisters each. The inhaler is packaged in a moisture-protective foil tray with a desiccant and a peelable lid (NDC 0173-0869-10).
ANORO ELLIPTA is also supplied in an institutional pack of a disposable light grey and red plastic inhaler containing 2 double-foil blister strips with 7 blisters each. The inhaler is packaged in a moisture protective foil tray with a desiccant and a peelable lid (NDC 0173-0869-06).
Store at room temperature between 68°F and 77°F (20°C and 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Store in a dry place away from direct heat or sunlight. Keep out of reach of children.
ANORO ELLIPTA should be stored inside the unopened moisture-protective foil tray and only removed from the tray immediately before initial use. Discard ANORO ELLIPTA 6 weeks after opening the foil tray or when the counter reads “0” (after all blisters have been used), whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Asthma-Related Death: Inform patients that LABA, such as vilanterol, one of the active ingredients in ANORO ELLIPTA, increase the risk of asthma-related death. ANORO ELLIPTA is not indicated for the treatment of asthma.
Not for Acute Symptoms: Inform patients that ANORO ELLIPTA is not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise them to treat acute symptoms with a rescue inhaler such as albuterol. Provide patients with such medicine and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
• Symptoms get worse
• Need for more inhalations than usual of their rescue inhaler
Patients should not stop therapy with ANORO ELLIPTA without physician/provider guidance since symptoms may recur after discontinuation.
Do Not Use Additional Long-Acting Beta2-Agonists: Instruct patients to not use other medicines containing a LABA. Patients should not use more than the recommended once-daily dose of ANORO ELLIPTA.
Instruct patients who have been taking inhaled, short-acting beta2-agonists on a regular basis to discontinue the regular use of these products and use them only for the symptomatic relief of acute symptoms.
Paradoxical Bronchospasm: As with other inhaled medicines, ANORO ELLIPTA can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue ANORO ELLIPTA.
Risks Associated With Beta-Agonist Therapy: Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness. Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Worsening of Narrow-Angle Glaucoma: Instruct patients to be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
Worsening of Urinary Retention: Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c

|













