|
近日,美国食品药品监督管理局批准Xuriden上市。Xuriden是第一款由FDA准许的用于治疗遗传性乳清酸尿症的药物。遗传性乳清酸尿症是一种罕见的代谢紊乱疾病,据报道全世界仅有20例左右的遗传性乳清酸尿症的患者。
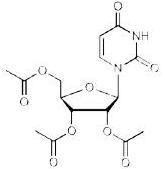
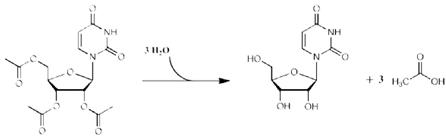
may use 1 entire 2 gram packet without weighing or measuring may use 2 entire 2 gram packets without weighing or measuring may use 3 entire 2 gram packets without weighing or measuring may use 4 entire 2 gram packets without weighing or measuring 2.2 Preparation and Administration Once the measured dose has been removed from the XURIDEN packet, discard the unused portion of granules. Do not use any granules left in the open packet. Administration with Food Place 3 to 4 ounces of applesauce, pudding or yogurt in a clean container. Mix the measured amount of granules in the applesauce, pudding or yogurt Swallow applesauce/pudding/yogurt immediately. Do not chew the granules. Do not save the applesauce/pudding/yogurt for later use. Drink at least 4 ounces of water. Administration in Milk or Infant Formula XURIDEN can be mixed with milk or infant formula instead of soft foods, as described above. For infants receiving up to ¼ teaspoon (0.8 grams) of XURIDEN. After weighing the dose of XURIDEN: Remove the syringe plunger from a pharmacy-provided oral 10 mL syringe. With a finger under the tip of the syringe, pour the measured amount of XURIDEN granules into the syringe barrel. Reinsert the syringe plunger. In a clean container with 10 mL of milk or infant formula, insert the tip of the oral syringe and draw up 5 mL of milk/infant formula into the syringe. Gently swirl the syringe in order to prevent the granules from settling. Administer the mixture immediately between the cheek and gum at the back of the mouth. Do not save the milk and granule mixture for later use. Refill the syringe with the remaining 5 mL of milk/infant formula, swirl gently, and administer. Follow this with a bottle of milk or infant formula if desired. For older children (age 3 years and older) and adults: Pour 3 to 4 ounces of milk into a clean container. Add the measured amount of granules into the milk and stir briefly Drink the mixture immediately. Do not chew the granules. Do not save the mixture for later use. Drink at least 4 more ounces of water or milk. 3 DOSAGE FORMS AND STRENGTHS Oral granules: 2 grams of orange-flavored oral granules (95% w/w) in single-use packets 4 CONTRAINDICATIONS None. 5 WARNINGS AND PRECAUTIONS None. 6 ADVERSE REACTIONS 6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions and using a wide range of doses, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of XURIDEN was assessed in 4 patients with hereditary orotic aciduria ranging in age from 3 to 19 years (3 male, 1 female) who received 60 mg/kg once daily of XURIDEN for six weeks. The patients continued to receive XURIDEN for at least 9 months at dosages of up to 120 mg/kg once daily. No adverse reactions were reported with XURIDEN. In clinical trials evaluating the use of XURIDEN for treatment of indications other than hereditary orotic aciduria, diarrhea was observed in some adult patients who received doses of 4 grams or 8 grams per day or greater [see Dosage and Administration (2.1)]. 8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Risk Summary There are no available data on XURIDEN use in pregnant women to inform a drug-associated risk. When administered orally to pregnant rats during the period of organogenesis, uridine triacetate at doses similar to the maximum recommended human dose (MRHD) of 240 mg/kg per day was not teratogenic and did not produce adverse effects on embryo-fetal development [see Data]. The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. Data Animal Data In an embryo-fetal development study, uridine triacetate was administered orally to pregnant rats during the period of organogenesis at doses up to 2000 mg/kg per day (about 1.3 times the maximum recommend human dose (MRHD) of 240 mg/kg per day on a body surface area basis). There was no evidence of teratogenicity or harm to the fetus and no effect on maternal body weight and overall health. 8.2 Lactation Risk Summary There are no data on the presence of uridine triacetate in human milk, the effect on the breastfed infant or the effect on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for XURIDEN and any potential adverse effects on the breastfed infant from XURIDEN or from the underlying maternal condition. 8.4 Pediatric Use The safety and effectiveness of XURIDEN have been established in pediatric patients. Use of XURIDEN is supported by a single open-label clinical trial of uridine triacetate in 4 patients and a retrospective review of the clinical course of 19 patients with hereditary orotic aciduria who were treated with uridine beginning at ages 2 months to 12 years. There are no apparent differences in clinical response between adults and pediatric patients with hereditary orotic aciduria treated with uridine, however, data are limited. [see Clinical Studies (14)] 11 DESCRIPTION XURIDEN (uridine triacetate) oral granules is a pyrimidine analog containing uridine triacetate. Uridine triacetate has the chemical designation (2',3',5'-tri-O-acetyl-ß-D-ribofuranosyl)-2,4(1H,3H)-pyrimidinedione. The molecular weight is 370.3 and it has an empirical formula of C15H18N2O9. The structural formula is:
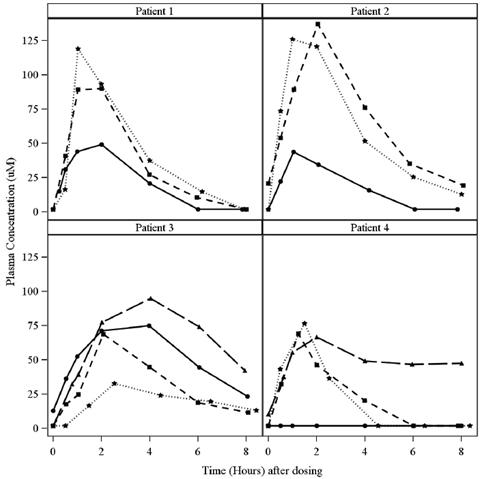
The dose of XURIDEN was increased on Day 116 to 120 mg/kg per day. Serial plasma samples were taken on Day 160 (44 days after the dose increase) for plasma uridine levels. Tmax range is expressed as the minimum and maximum values obtained Food Effect on Uridine PK: A study in healthy adult subjects receiving a slightly different formulation of uridine triacetate granules (6 gram dose) under fed and fasted conditions showed no difference in the overall rate and extent of uridine exposure. Distribution Circulating uridine is taken up into mammalian cells via specific nucleoside transporters, and also crosses the blood brain barrier. Excretion Uridine can be excreted via the kidneys, but is also metabolized by normal pyrimidine catabolic pathways present in most tissues. Drug Interaction Studies In vitro enzyme inhibition data did not reveal meaningful inhibitory effects of uridine triacetate or uridine on CYP3A4, CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1. In vitro e nzyme induction data did not reveal an inducing effect of XURIDEN or uridine on CYP1A2, CYP2B6, or CYP3A4. In vitro data showed that uridine triacetate was a weak substrate for P-glycoprotein. Uridine triacetate inhibited the transport of a known P-glycoprotein substrate, digoxin with an IC50 of 344 μM. Due to potential for high local (gut) concentrations of the drug after dosing, interaction of Xuriden with orally administered P-gp substrate drugs cannot be ruled out. In vivo data in humans are not available. 13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility Long-term studies in animals have not been performed to evaluate the carcinogenic potential of uridine triacetate. Uridine triacetate was not genotoxic in the Ames test, the mouse lymphoma assay and the mouse micronucleus test. Orally administered uridine triacetate did not affect fertility or general reproductive performance in male and female rats at doses up to 2000 mg/kg per day (about 1.3 times the maximum recommend human dose (MRHD) of 240 mg/kg per day on a body surface area basis). 14 CLINICAL STUDIES The efficacy of XURIDEN was evaluated in an open-label study in 4 patients with hereditary orotic aciduria (3 male, 1 female; age range from 3 to 19 years). Three patients were previously treated with uridine and were switched at study entry to XURIDEN. All patients were administered XURIDEN orally at a daily dosage of 60 mg/kg once daily. The study duration was 6 weeks. The study assessed changes in the patients' pre-specified hematologic parameter during the 6-week trial period. The pre-specified hematologic parameters were: percent neutrophils (Patient 1), white blood cell count (Patient 2), and mean corpuscular volume (Patients 3 and 4). For patients switched from oral uridine to oral XURIDEN (Patients 1, 2, and 3), the primary endpoint was stability of the hematologic parameter; for the treatment-naïve patient (Patient 4), the primary endpoint was improvement of the hematologic parameter. Secondary endpoints were urine orotic acid and orotidine levels, and growth (height and weight). After six weeks of treatment, hematologic values remained stable for all 4 patients. Table 6 summarizes the primary efficacy results. Table 6: Primary Efficacy Results for Study 1
During an extension phase of the trial, patients continued to receive uridine triacetate. Dosing during the extension phase ranged from 60 mg/kg to 120 mg/kg once daily. After 6 months of treatment, Patient #1's neutrophil percent value normalized; hematologic parameters for the other three patients remained stable. Orotic acid and orotidine levels also remained stable for all four patients. The treatment effect of XURIDEN on growth was assessed in the three pediatric patients (Patients 1, 3, and 4). At baseline, weight and height measurements were at or below the lower limit of normal for age (<5th percentile for age) for Patients 1 and 4; height and weight measurements were within the normal range for age for Patient 3. After 6 months of treatment, Patients 1 and 3 experienced improved weight growth, as reflected in increases in their weight-for-age percentiles and weight velocity percentiles; Patient 4's weight growth remained stable (i.e., weight percentile for age and weight velocity percentile for age was unchanged). Height growth remained stable in all three patients (i.e., height percentiles for age and height velocity percentiles for age were unchanged). INSERT 12 MONTH GROWTH RESULTS Case reports Nineteen (19) case reports of patients with HOA have been documented in published literature. Eighteen (18) were diagnosed as infants or children between the ages of 2 months and 12 years, and one was diagnosed at age 28. All 19 patients presented with significantly elevated levels of urinary orotic acid. Fifteen of 19 had abnormal hematologic parameters at presentation, including 15 with megaloblastic anemia, 8 with leukopenia and at least 2 with neutropenia. Oral administration of exogenous sources of uridine was reported to significantly improve hematologic abnormalities (megaloblastic anemia, leukopenia and neutropenia) within 2 to 3 weeks in almost all documented cases when administered in sufficient amounts. Concentrations of urinary orotic acid were significantly reduced within 1 to 2 weeks of initiating uridine replacement therapy. Some fluctuation in levels of urinary orotic acid were observed, but always at much lower levels than those reported prior to treatment. Improvements in body weight and other developmental parameters were also documented over time with continued uridine replacement therapy. The effects of exogenous uridine were maintained over months and years, as long as treatment continued at sufficient doses (with appropriate dose increases based on body weight increases). Within days up to 2 or 3 weeks, most hematologic abnormalities and orotic aciduria reappeared when administration of uridine was stopped or doses reduced. If treatment was interrupted for longer periods, body weight and other gains receded. If absolute dosages were not adjusted adequately to compensate for body weight gains, signs and symptoms of HOA recurred. 16 HOW SUPPLIED/STORAGE AND HANDLING XURIDEN orange-flavored oral granules (95% w/w) are available in single-use packets (NDC 69468-152-02) containing 2 grams of uridine triacetate in cartons of 30 packets each (NDC 69468-152-30) of uridine triacetate in cartons of 30 packets each. Store at controlled room temperature, 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). 17 PATIENT COUNSELING INFORMATION Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information) Advise the patient or caregiver to measure the prescribed dose using either a balance accurate to at least 0.1 gram, or a pharmacy-provided graduated teaspoon, accurate to the fraction of the dose to be administered. For some doses 1 or more entire 2 gram packets can be used without weighing out the granules. [see Dosage and Administration (2.1)]. Advise the patient or caregiver that XURIDEN can be taken without regard to meals. Advise the patient or caregiver to discard the unused portion of granules in a packet after measuring out the dose. Advise the patient or caregiver to not use any granules left in the open packet. Advise the patient or caregiver that XURDEN can be taken mixed in applesauce, pudding or yogurt, or mixed in milk or water. The dose should be taken immediately after mixing and should not be saved for later use. Advise the patient or caregiver not to chew the granules and to drink a glass of water after swallowing the mixture. [see Preparation and Administration (2.2)]. Advise the caregiver of an infant patient that XURIDEN can be mixed with milk or infant formula and given using a pharmacy-provided 10 mL syringe. If desired, administration can be followed by a bottle of milk or infant formula. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Xuriden(uridine triacetate)尿苷三乙酸口服颗粒剂简介:
近日,美国食品药品监督管理局批准Xuriden上市。Xuriden是第一款由FDA准许的用于治疗遗传性乳清酸尿症的药物。遗传性乳清酸尿症是一种罕见的代谢紊乱疾病,据报道全世界仅有20例左右的遗传性乳清酸尿症 ... 责任编辑:admin |
最新文章更多推荐文章更多热点文章更多
|