英文药名:TAKELDA(Aspirin/Lansoprazole Combination Tablets)
中文药名:复方阿司匹林兰索拉唑片
生产厂家:武田薬品
タケルダ配合錠
治疗类别名称
阿司匹林/兰索拉唑制剂
药品成分:阿司匹林100mg; 兰索拉唑15mg
批准上市时间:2014年6月
商標名
TAKELDA Combination Tablets
阿司匹林
化学構造式
一般名
アスピリン(Aspirin)〔JAN〕
化学名
2-Acetoxybenzoic acid
分子式
C9H8O4
分子量
180.16
融点
約136℃
性状
阿司匹林是白色结晶,用谷物或粉末,无臭,稍有一个酸味。乙醇(95)或溶于丙酮,微溶于乙醚,微溶于水。溶于氢氧化钠TS或碳酸钠试剂。它变得水杨酸和乙酸在潮湿的空气中逐渐水解。
兰索拉唑
化学構造式
一般名
ランソプラゾール(Lansoprazole)〔JAN〕
化学名
(RS)-2-({[3-Methyl-4-(2, 2, 2-trifluoroethoxy)-2-pyridyl]methyl}sulfinyl)benzimidazole
分子式
C16H14F3N3O2S
分子量
369.36
融点
約166℃(分解)
性状
兰索拉唑是白色结晶性粉末,以棕白色带。 N,N-二甲基甲酰胺,以更可溶,容易微溶于甲醇,乙醇(99.5),以微溶,在乙醚中极微溶,并且几乎不溶于水。
药效药理
1. 阿司匹林
阿司匹林因为它会导致血小板环氧合酶-1(COX-1)活性的抑制在低剂量,显示出抑制血小板聚集的抑制作用血栓素A2的产生。从对血小板COX-1阿司匹林的本效果持续的不可逆的血小板为7至10天,和阿司匹林的血小板功能的重复施用的寿命累积抑制的事实,显示了血栓栓塞的抑制作用。
2. 兰索拉唑
兰索拉唑是过渡到胃粘膜壁细胞的形成酸的现场后,通过与酸的转移反应是结构转化为活性体,发挥作用作为质子泵此酸转移产物被定位成酸生产现场及和H +,结合到SH基团K+-ATP酶的,通过抑制酶的活性被认为是抑制胃酸分泌。
适用症:
・ 心绞痛(慢性稳定型心绞痛,不稳定型心绞痛),心肌梗塞,缺血性脑血管病(短暂性脑缺血发作(TIA),脑梗塞)
・ 冠状动脉旁路移植术施行后(CABG)或经皮腔内冠状动脉成形术(PTCA)
用量和用法
成人每日一次,每次1片。
包装规格:
100片/盒(10片×10)
140片/盒(14片×10)
制造厂商
武田化学工业有限公司
原处方资料附件:http://www.info.pmda.go.jp/go/pack/3399102F1026_1_01/
TAKELDA Combination Tablets(タケルダ配合錠)
Brand name : TAKELDA Combination Tablets
Active ingredient: Aspirin
Lansoprazole
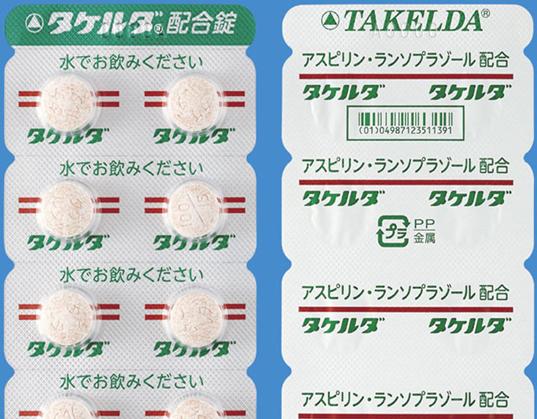
Dosage form: white to yellowish white tablet with redbrown to dark-brown flecks, diameter 10.0 mm, thickness 5.4 mm
Print on wrapping: (face) タケルダ, 水でお飲みください (back) タケルダ, アスピリン・ランソプラゾール配合
Effects of this medicine
Aspirin inhibits activities of platelet cyclooxygenase-1 to suppress platelet aggregation and eventually delay or prevent blood coagulation while lansoprazole inhibits the enzymatic activity of proton pump in the gastric mucosa to suppress gastric acid secretion.
Aspirin prevents obstruction of a blood vessel by a blood clot that has formed but may cause gastric or duodenal ulcers; therefore, lansoprazole prevents such untoward events.
It is usually used to suppress thrombus/embolus formation due to angina pectoris, myocardial infarction, or ischemic cerebrovascular disorder or to suppress thrombus/embolus formation after coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA) in patients with a history of gastric or duodenal ulcers.
Before using this medicine, be sure to tell your doctor and pharmacist
•If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have peptic ulcer, hematological disorder or a history of hematological disorder, bleeding tendency or predisposition to bleeding tendency, bronchial asthma or a history of bronchial asthma, liver disorder or a history of liver disorder, renal disorder or a history of renal disorder.
If you drink alcohol on a regular basis.
If you are scheduled for surgery, cardiac catheter test or tooth extraction.
•If you are pregnant or breastfeeding.
•If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Dosing schedule (How to take this medicine)
•Your dosing schedule prescribed by your doctor is <<to be written by a healthcare professional>>
•For adults: In general, take 1 tablet at a time, once a day. Strictly follow the instructions.
•This medicine cannot be dissolved in the mouth. Take the medicine with water without chewing or breaking it into pieces.
•If you miss a dose, take the missed dose as soon as possible. You should never take two doses at one time.
•If you accidentally take more than your prescribed dose, consult with your doctor or pharmacist.
•Do not stop taking this medicine unless your doctor instructs you to do so.
Precautions while taking this medicine
•This medicine may induce or enhance gastrointestinal bleeding; therefore, avoid taking this medicine with any alcoholic beverage.
Possible adverse reactions to this medicine
The most commonly reported adverse reactions include constipation, diarrhea, rash, itching, polymorphic erythema, swelling, anemia, platelet hypofunction (prolonged bleeding time), enlarged feeling of abdomen, stomatitis, colitis, headache, dizziness, excitement, conjunctivitis, gynecomastia, malaise, over breathing, and hypoglycemia. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
•generalized rash, facial swelling, breathlessness [anaphylaxis, shock]
•dullness, fever, shortness of breath, nasal bleeding/subcutaneous hemorrhage, red-brown urine [pancytopenia, agarnulocytosis, aplastic anemia, granulocytopenia, hemolytic anemia, decreased platelet count, anemia]
•yellowing of the skin or whites of the eyes, dullness, loss of appetite [serous liver dysfunction]
•fever, general malaise, eruptions on/reddening of the skin and eyes and eruptions/reddening in the mouth, exfoliation of skin [toxic epidermal necrolysis, mucocutaneous-ocular syndrome, exfoliative dermatitis]
•fever, dry cough, breathlessness [interstitial pneumonia]
•fever, skin eruption, joint pain [interstitial nephritis]
•headache, nausea/vomiting, disturbance of consciousness, motor paralysis, bloody phlegm, bloody stool, nose bleeding, vision disorder [intracranial bleeding, pulmonary hemorrhage, gastrointestinal hemorrhage, epistaxis, ocular fundus bleeding]
•breathlessness, whistling sound [asthmatic attack]
•heartburn, heavy feeling of stomach, back pain, abdominal pain, diarrhea, bloody stool [peptic ulcer, small/large intestine ulcer]
The above symptoms do not describe all the adverse reactions to this medicine. Consult with your doctor or pharmacist if you notice any symptoms of concern other than those listed above.
Storage conditions and other information
•Keep out of the reach of children. Store away from direct sunlight, heat and moisture.
•Discard the remainder. Do not store them.
Takeda Chemical Industries, Ltd.Internal
Published: 6/2014
The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.



